An extensive study on multiple ETL and HTL layers to design and simulation of high-performance lead-free CsSnCl3-based perovskite solar cells
- PMID: 36781884
- PMCID: PMC9925818
- DOI: 10.1038/s41598-023-28506-2
An extensive study on multiple ETL and HTL layers to design and simulation of high-performance lead-free CsSnCl3-based perovskite solar cells
Abstract
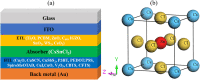
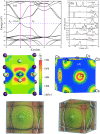
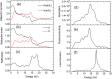
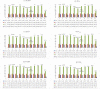
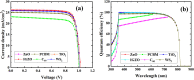
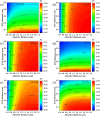
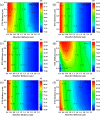
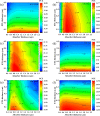
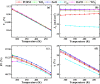
Cesium tin chloride (CsSnCl3) is a potential and competitive absorber material for lead-free perovskite solar cells (PSCs). The full potential of CsSnCl3 not yet been realized owing to the possible challenges of defect-free device fabrication, non-optimized alignment of the electron transport layer (ETL), hole transport layer (HTL), and the favorable device configuration. In this work, we proposed several CsSnCl3-based solar cell (SC) configurations using one dimensional solar cell capacitance simulator (SCAPS-1D) with different competent ETLs like indium-gallium-zinc-oxide (IGZO), tin-dioxide (SnO2), tungsten disulfide (WS2), ceric dioxide (CeO2), titanium dioxide (TiO2), zinc oxide (ZnO), C60, PCBM, and HTLs of cuprous oxide (Cu2O), cupric oxide (CuO), nickel oxide (NiO), vanadium oxide (V2O5), copper iodide (CuI), CuSCN, CuSbS2, Spiro MeOTAD, CBTS, CFTS, P3HT, PEDOT:PSS. Simulation results revealed that ZnO, TiO2, IGZO, WS2, PCBM, and C60 ETLs-based halide perovskites with ITO/ETLs/CsSnCl3/CBTS/Au heterostructure exhibited outstanding photoconversion efficiency retaining nearest photovoltaic parameters values among 96 different configurations. Further, for the six best-performing configurations, the effect of the CsSnCl3 absorber and ETL thickness, series and shunt resistance, working temperature, impact of capacitance, Mott-Schottky, generation and recombination rate, current-voltage properties, and quantum efficiency on performance were assessed. We found that ETLs like TiO2, ZnO, and IGZO, with CBTS HTL can act as outstanding materials for the fabrication of CsSnCl3-based high efficiency (η ≥ 22%) heterojunction SCs with ITO/ETL/CsSnCl3/CBTS/Au structure. The simulation results obtained by the SCAPS-1D for the best six CsSnCl3-perovskites SC configurations were compared by the wxAMPS (widget provided analysis of microelectronic and photonic structures) tool for further validation. Furthermore, the structural, optical and electronic properties along with electron charge density, and Fermi surface of the CsSnCl3 perovskite absorber layer were computed and analyzed using first-principle calculations based on density functional theory. Thus, this in-depth simulation paves a constructive research avenue to fabricate cost-effective, high-efficiency, and lead-free CsSnCl3 perovskite-based high-performance SCs for a lead-free green and pollution-free environment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Saparov B, et al. Thin-film deposition and characterization of a Sn-deficient perovskite derivative Cs2SnI6. Chem. Mater. 2016;28:2315–2322. doi: 10.1021/acs.chemmater.6b00433. - DOI
-
- Qiu X, et al. From unstable CsSnI3 to air-stable Cs2SnI6: A lead-free perovskite solar cell light absorber with bandgap of 1.48 eV and high absorption coefficient. Sol. Energy Mater. Sol. Cells. 2017;159:227–234. doi: 10.1016/j.solmat.2016.09.022. - DOI
LinkOut - more resources
Full Text Sources

