Reversal of aging-associated increase in myelopoiesis and expression of alarmins by angiotensin-(1-7)
- PMID: 36782016
- PMCID: PMC9925828
- DOI: 10.1038/s41598-023-29853-w
Reversal of aging-associated increase in myelopoiesis and expression of alarmins by angiotensin-(1-7)
Abstract
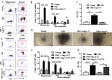
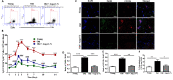
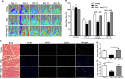
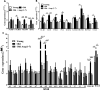
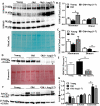
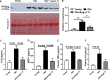
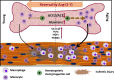
Aging is associated with chronic systemic inflammation largely due to increased myelopoiesis, which in turn increases risk for vascular disease. We have previously shown evidence for the therapeutic potential of Angiotensin-(1-7) (Ang-(1-7)) in reversing vasoreparative dysfunction in aging. This study tested the hypothesis that ischemic vascular repair in aging by Ang-(1-7) involves attenuation of myelopoietic potential in the bone marrow and decreased mobilization of inflammatory cells. Young or Old male mice of age 3-4 and 22-24 months, respectively, received Ang-(1-7) (1 µg/kg/min, s.c.) for four weeks. Myelopoiesis was evaluated in the bone marrow (BM) cells by carrying out the colony forming unit (CFU-GM) assay followed by flow cytometry of monocyte-macrophages. Expression of pro-myelopoietic factors and alarmins in the hematopoietic progenitor-enriched BM cells was evaluated. Hindlimb ischemia (HLI) was induced by femoral ligation, and mobilization of monocytes into the blood stream was determined. Blood flow recovery was monitored by Laser Doppler imaging and infiltration of inflammatory cells was evaluated by immunohistochemistry. BM cells from Old mice generated a higher number of monocytes (Ly6G-CD11b+Ly6Chi) and M1 macrophages (Ly6ChiF4/80+) compared to that of Young, which was reversed by Ang-(1-7). Gene expression of selected myelopoietic factors, alarmins (S100A8, S100A9, S100A14 and HMGb1) and the receptor for alarmins, RAGE, was higher in the Old hematopoietic progenitor-enriched BM cells compared to the Young. Increased expressions of these factors were decreased by Ang-(1-7). Ischemia-induced mobilization of monocytes was higher in Old mice with decreased blood flow recovery and increased infiltration of monocyte-macrophages compared to the Young, all of which were reversed by Ang-(1-7). Enhanced ischemic vascular repair by Ang-(1-7) in aging is largely by decreasing the generation and recruitment of inflammatory monocyte-macrophages to the areas of ischemic injury. This is associated with decreased alarmin signaling in the BM-hematopoietic progenitor cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

