Innate immune inflammatory cell death: PANoptosis and PANoptosomes in host defense and disease
- PMID: 36782083
- PMCID: PMC10423303
- DOI: 10.1002/eji.202250235
Innate immune inflammatory cell death: PANoptosis and PANoptosomes in host defense and disease
Abstract
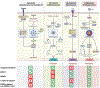
Regulated cell death (RCD) triggered by innate immune activation is an important strategy for host survival during pathogen invasion and perturbations of cellular homeostasis. There are two main categories of RCD, including nonlytic and lytic pathways. Apoptosis is the most well-characterized nonlytic RCD, and the inflammatory pyroptosis and necroptosis pathways are among the best known lytic forms. While these were historically viewed as independent RCD pathways, extensive evidence of cross-talk among their molecular components created a knowledge gap in our mechanistic understanding of RCD and innate immune pathway components, which led to the identification of PANoptosis. PANoptosis is a unique innate immune inflammatory RCD pathway that is regulated by PANoptosome complexes upon sensing pathogens, pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs) or the cytokines produced downstream. Cytosolic innate immune sensors and regulators, such as ZBP1, AIM2 and RIPK1, promote the assembly of PANoptosomes to drive PANoptosis. In this review, we discuss the molecular components of the known PANoptosomes and highlight the mechanisms of PANoptosome assembly, activation and regulation identified to date. We also discuss how PANoptosomes and mutations in PANoptosome components are linked to diseases. Given the impact of RCD, and PANoptosis specifically, across the disease spectrum, improved understanding of PANoptosomes and their regulation will be critical for identifying new therapeutic targets and strategies.
Keywords: Apoptosis; Caspases; Inflammasome; Necroptosis; PANoptosis; PANoptosome; Pyroptosis.
© 2023 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest statement
T.-D.K. is a consultant for Pfizer. All other authors have no commercial or financial conflicts of interest.
Figures

References
-
- Nagata S and Tanaka M, Programmed cell death and the immune system. Nat Rev Immunol 2017. 17: 333–340. - PubMed
-
- Ivanisenko NV, Seyrek K, Hillert-Richter LK, König C, Espe J, Bose K and Lavrik IN, Regulation of extrinsic apoptotic signaling by c-FLIP: towards targeting cancer networks. Trends Cancer 2022. 8: 190–209. - PubMed
-
- Martinon F, Burns K and Tschopp J, The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002. 10: 417–426. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

