Heavily treatment-experienced people living with HIV in the OPERA® cohort: population characteristics and clinical outcomes
- PMID: 36782125
- PMCID: PMC9926692
- DOI: 10.1186/s12879-023-08038-w
Heavily treatment-experienced people living with HIV in the OPERA® cohort: population characteristics and clinical outcomes
Abstract
Background: Multi-class resistance, intolerance, and drug-drug interactions can result in unique antiretroviral (ART) combinations for heavily treatment-experienced (HTE) people living with HIV (PLWH). We aimed to compare clinical outcomes between HTE and non-HTE PLWH.
Methods: Eligible ART-experienced PLWH in care in the OPERA® Cohort were identified in a cross-sectional manner on December 31, 2016 and observed from the date of initiation of the ART regimen taken on December 31, 2016 until loss to follow up, death, study end (December 31, 2018), or becoming HTE (non-HTE group only). In the absence of resistance data, HTE was defined based on the ART regimens used (i.e., exposed to ≥ 3 core agent classes or regimen suggestive of HTE). Time to virologic undetectability, failure, and immunologic preservation were assessed using Kaplan-Meier methods; cumulative probabilities were compared between the two groups. Regimen changes, incident morbidities, and death were described.
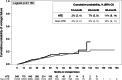
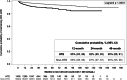
Results: A total of 24,183 PLWH (2277 HTE PLWH, 21,906 non-HTE) were followed for a median of 28 months (IQR 21, 38). Viremic HTE PLWH (viral load [VL] ≥ 50 copies/mL) were less likely to achieve undetectability (VL < 50 copies/mL; 24-month cumulative probability: 80% [95% Confidence Interval 77-82]) than their non-HTE counterparts (85% [84-86]). No difference was observed in the probability of maintaining VLs < 200 copies/mL over the first 48 months after achieving suppression (< 50 copies/mL). HTE PLWH were less likely than non-HTE PLWH to maintain CD4 cell counts ≥ 200 cells/µL (24-month cumulative probability: 95% HTE [91-93]; 97% non-HTE [97-97]), and more likely to change regimens (45% HTE; 41% non-HTE). Incident non-AIDS defining event (ADE) morbidities were common in both populations, though more likely among HTE PLWH (45%) than non-HTE PLWH (35%). Incident ADE morbidities and deaths were uncommon among HTE (ADEs 5%; deaths 2%) and non-HTE (ADEs 2%; deaths 1%) PLWH.
Conclusions: HTE PLWH were at greater risk of unfavorable treatment outcomes than non-HTE PLWH, suggesting additional therapeutic options are needed for this vulnerable population.
Keywords: ART; Characteristics; HIV; HTE; Outcomes; Prevalence.
© 2023. The Author(s).
Conflict of interest statement
RKH has received research Grants from Gilead Sciences and Janssen, speaker honoraria and advisory boards from ViiV Healthcare, Merck, Gilead Sciences and Janssen, and advisory board participation with ViiV, Gilead Sciences, Janssen, and Epividian. CEH, VV and AC are employed by ViiV Healthcare and hold stocks and shares in GSK as part of their employment. JSF, LB, and GPF are employed by Epividian, Inc.; Epividian has had research funded by ViiV Healthcare, Merck & Co., Janssen Pharmaceutica, Gilead Sciences, TheraTechnologies, EMD Serono, and AIDS Healthcare Foundation. PCL and GP receive honoraria for advisory board participation with Epividian.
Figures


References
-
- WHO Expert Consultation. ART failure and strategies for switching ART regimens in the WHO European region. Copenhagen. 2007.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

