Plasma lipidomic profiling reveals metabolic adaptations to pregnancy and signatures of cardiometabolic risk: a preconception and longitudinal cohort study
- PMID: 36782297
- PMCID: PMC9926745
- DOI: 10.1186/s12916-023-02740-x
Plasma lipidomic profiling reveals metabolic adaptations to pregnancy and signatures of cardiometabolic risk: a preconception and longitudinal cohort study
Abstract
Background: Adaptations in lipid metabolism are essential to meet the physiological demands of pregnancy and any aberration may result in adverse outcomes for both mother and offspring. However, there is a lack of population-level studies to define the longitudinal changes of maternal circulating lipids from preconception to postpartum in relation to cardiometabolic risk factors.
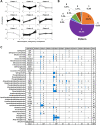
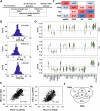
Methods: LC-MS/MS-based quantification of 689 lipid species was performed on 1595 plasma samples collected at three time points in a preconception and longitudinal cohort, Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO). We mapped maternal plasma lipidomic profiles at preconception (N = 976), 26-28 weeks' pregnancy (N = 337) and 3 months postpartum (N = 282) to study longitudinal lipid changes and their associations with cardiometabolic risk factors including pre-pregnancy body mass index, body weight changes and glycaemic traits.
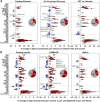
Results: Around 56% of the lipids increased and 24% decreased in concentration in pregnancy before returning to the preconception concentration at postpartum, whereas around 11% of the lipids went through significant changes in pregnancy and their concentrations did not revert to the preconception concentrations. We observed a significant association of body weight changes with lipid changes across different physiological states, and lower circulating concentrations of phospholipids and sphingomyelins in pregnant mothers with higher pre-pregnancy BMI. Fasting plasma glucose and glycated haemoglobin (HbA1c) concentrations were lower whereas the homeostatic model assessment of insulin resistance (HOMA-IR), 2-h post-load glucose and fasting insulin concentrations were higher in pregnancy as compared to both preconception and postpartum. Association studies of lipidomic profiles with these glycaemic traits revealed their respective lipid signatures at three physiological states. Assessment of glycaemic traits in relation to the circulating lipids at preconception with a large sample size (n = 936) provided an integrated view of the effects of hyperglycaemia on plasma lipidomic profiles. We observed a distinct relationship of lipidomic profiles with different measures, with the highest percentage of significant lipids associated with HOMA-IR (58.9%), followed by fasting insulin concentration (56.9%), 2-h post-load glucose concentration (41.8%), HbA1c (36.7%), impaired glucose tolerance status (31.6%) and fasting glucose concentration (30.8%).
Conclusions: We describe the longitudinal landscape of maternal circulating lipids from preconception to postpartum, and a comprehensive view of trends and magnitude of pregnancy-induced changes in lipidomic profiles. We identified lipid signatures linked with cardiometabolic risk traits with potential implications both in pregnancy and postpartum life. Our findings provide insights into the metabolic adaptations and potential biomarkers of modifiable risk factors in childbearing women that may help in better assessment of cardiometabolic health, and early intervention at the preconception period.
Trial registration: ClinicalTrials.gov, NCT03531658.
Keywords: Cardiometabolic risk; Glucose homeostasis; Insulin resistance; Lipidomics; Metabolic adaptations; Postpartum; Preconception; Pregnancy; Weight changes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Okala SG, Sise EA, Sosseh F, Prentice AM, Woollett LA, Moore SE. Maternal plasma lipid levels across pregnancy and the risks of small-for-gestational age and low birth weight: a cohort study from rural Gambia. BMC Pregnancy Childbirth. 2020;20(1):153. doi: 10.1186/s12884-020-2834-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

