The activation of EP300 by F11R leads to EMT and acts as a prognostic factor in triple-negative breast cancers
- PMID: 36782375
- PMCID: PMC10073929
- DOI: 10.1002/cjp2.313
The activation of EP300 by F11R leads to EMT and acts as a prognostic factor in triple-negative breast cancers
Abstract
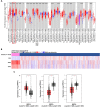
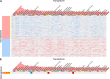
Cancer progression is influenced by junctional adhesion molecule (JAM) family members. The relationship between JAM family members and different types of cancer was examined using The Cancer Genome Atlas dataset. mRNA levels of the F11R (F11 receptor) in tumours were inversely correlated to the expression of JAM-2 and JAM-3. This relationship was unique to breast cancer (BCa) and was associated with poor prognosis (p = 0.024, hazard ratio = 1.44 [1.05-1.99]). A 50-gene molecular signature (prediction analysis of microarray 50) was used to subtype BCa. F11R mRNA expression significantly increased in human epidermal growth factor receptor 2 (HER2)-enriched (p = 0.0035) and basal-like BCa tumours (p = 0.0005). We evaluated F11R protein levels in two different compositions of BCa subtype patient tissue array cohorts to determine the relationship between BCa subtype and prognosis. Immunohistochemistry staining revealed that a high F11R protein level was associated with poor overall survival (p < 0.001; Taipei Medical University [TMU] cohort, p < 0.001; Kaohsiung Veterans General Hospital [KVGH] cohort) or disease-free survival (p < 0.001 [TMU cohort], p = 0.034 [KVGH cohort]) in patients with BCa. Comparison of F11R levels in different subtypes revealed the association of poor prognosis with high levels of F11R among luminal (p < 0.001 [TMU cohort], p = 0.027 [KVGH cohort]), HER2 positive (p = 0.018 [TMU cohort], p = 0.037 [KVGH cohort]), and triple-negative (p = 0.013 [TMU cohort], p = 0.037 [KVGH cohort]) BCa. F11R-based RNA microarray analysis and Ingenuity Pathway Analysis were successful in profiling the detailed gene ontology of triple-negative BCa cells regulated by F11R. The EP300 transcription factor was highly correlated with F11R in BCa (R = 0.51, p < 0.001). By analysing these F11R-affected molecules with the L1000CDs datasets, we were able to predict some repurposing drugs for potential application in F11R-positive BCa treatment.
Keywords: EP300; F11R; breast cancer; survival curve; transcriptomics.
© 2023 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland and John Wiley & Sons Ltd.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–752. - PubMed
-
- Harbeck N, Penault‐Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers 2019; 5: 66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

