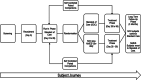
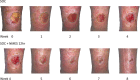
The impact of a new intervention for venous leg ulcers: A within-patient controlled trial
- PMID: 36785909
- PMCID: PMC10333027
- DOI: 10.1111/iwj.14107
The impact of a new intervention for venous leg ulcers: A within-patient controlled trial
Abstract
A major obstacle to the development of new treatments for venous leg ulcers is the difficulty in generating evidence for their effectiveness. Randomised controlled trials using complete healing as the endpoint are seldom powered to be successful, owing to the heterogeneity of cohorts. A novel approach to the evaluation of treatments is presented, using a self-controlled trial model and two metrics of short-term healing rate as alternate endpoints: rate of wound margin advance, and percentage area reduction over 4 weeks. Two different treatment regimens are compared: multi-layer compression alone, versus multi-layer compression combined with activation of the venous leg pump by neuromuscular stimulation. With 60 patients, adding neuromuscular stimulation to multilayer compression resulted in a significant two-fold increase in the rate of wound healing over a 4-week period, both in terms of wound margin advance and in terms of percentage area reduction. The use of these short-term intermediate endpoint metrics together with a self-controlled study design offers potential for distinguishing between the relative efficacies of interventions more rapidly, with greater sensitivity, and with fewer subjects than a conventional RCT cohort model.
Keywords: NMES; PAR; WMA; alternate healing endpoint; self-controlled trial; venous leg ulcers.
© 2023 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures
References
-
- Neumann HAM, Cornu‐Thenard A, Junger M, et al. Evidence‐based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J Eur Acad Dermatol Venereol. 2016;30:1843‐1875. - PubMed
-
- Harding KG. Chronic wounds: a clinical problem requiring ownership and coordination. Br J Dermatol. 2022;187:134‐135. - PubMed
-
- Phillips TJ, Dover JS. Leg ulcers. J Am Acad Dermatol. 1991;25:965‐987. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical