In Vitro and In Vivo Development of a β-Lactam-Metallo-β-Lactamase Inhibitor: Targeting Carbapenem-Resistant Enterobacterales
- PMID: 36786013
- PMCID: PMC10012271
- DOI: 10.1021/acsinfecdis.2c00485
In Vitro and In Vivo Development of a β-Lactam-Metallo-β-Lactamase Inhibitor: Targeting Carbapenem-Resistant Enterobacterales
Abstract
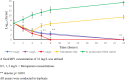
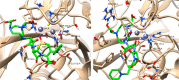
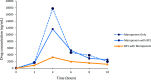
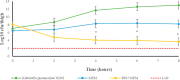
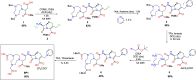
β-lactams are the most prescribed class of antibiotics due to their potent, broad-spectrum antimicrobial activities. However, alarming rates of antimicrobial resistance now threaten the clinical relevance of these drugs, especially for the carbapenem-resistant Enterobacterales expressing metallo-β-lactamases (MBLs). Antimicrobial agents that specifically target these enzymes to restore the efficacy of last resort β-lactam drugs, that is, carbapenems, are therefore desperately needed. Herein, we present a cyclic zinc chelator covalently attached to a β-lactam scaffold (cephalosporin), that is, BP1. Observations from in vitro assays (with seven MBL expressing bacteria from different geographies) have indicated that BP1 restored the efficacy of meropenem to ≤ 0.5 mg/L, with sterilizing activity occurring from 8 h postinoculation. Furthermore, BP1 was nontoxic against human hepatocarcinoma cells (IC50 > 1000 mg/L) and exhibited a potency of (Kiapp) 24.8 and 97.4 μM against Verona integron-encoded MBL (VIM-2) and New Delhi metallo β-lactamase (NDM-1), respectively. There was no inhibition observed from BP1 with the human zinc-containing enzyme glyoxylase II up to 500 μM. Preliminary molecular docking of BP1 with NDM-1 and VIM-2 sheds light on BP1's mode of action. In Klebsiella pneumoniae NDM infected mice, BP1 coadministered with meropenem was efficacious in reducing the bacterial load by >3 log10 units' postinfection. The findings herein propose a favorable therapeutic combination strategy that restores the activity of the carbapenem antibiotic class and complements the few MBL inhibitors under development, with the ultimate goal of curbing antimicrobial resistance.
Keywords: Klebsiella pneumoniae; New Delhi metallo β-lactamase; Verona integron-encoded MBL; carbapenem-resistant Enterobacterales; human hepatocarcinoma cells; β-Lactam-Metallo-β-Lactamase Inhibitor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
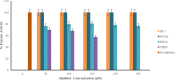
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

