Interconnections of Pseudomonas aeruginosa Quorum-Sensing Systems in Intestinal Permeability and Inflammation
- PMID: 36786582
- PMCID: PMC10127598
- DOI: 10.1128/mbio.03524-22
Interconnections of Pseudomonas aeruginosa Quorum-Sensing Systems in Intestinal Permeability and Inflammation
Abstract
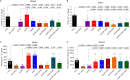
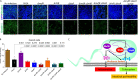
Quorum sensing (QS) is a highly conserved microbial communication mechanism based on the production and sensing of secreted signaling molecules. The recalcitrant pathogen Pseudomonas aeruginosa is a problematic nosocomial pathogen with complex interconnected QS systems controlling multiple virulence functions. The relevance of QS in P. aeruginosa pathogenesis is well established; however, the regulatory interrelationships of the three major QS systems, LasR/LasI, MvfR (PqsR)/PqsABCD, and RhlR/RhlI, have been studied primarily in vitro. It is, therefore, unclear how these relationships translate to the host environment during infection. Here, we use a collection of P. aeruginosa QS mutants of the three major QS systems to assess the interconnections and contributions in intestinal inflammation and barrier function in vivo. This work reveals that MvfR, not LasR or RhlR, promotes intestinal inflammation during infection. In contrast, we find that P. aeruginosa-driven murine intestinal permeability is controlled by an interconnected QS network involving all three regulators, with MvfR situated upstream of LasR and RhlR. This study demonstrates the importance of understanding the interrelationships of the QS systems during infection and provides critical insights for developing successful antivirulence strategies. Moreover, this work provides a framework to interrogate QS systems in physiologically relevant settings. IMPORTANCE Pseudomonas aeruginosa is a common multidrug-resistant bacterial pathogen that seriously threatens critically ill and immunocompromised patients. Intestinal colonization by this pathogen is associated with elevated mortality rates. Disrupting bacterial communication is a desirable anti-infective approach since these systems coordinate multiple acute and chronic virulence functions in P. aeruginosa. Here, we investigate the role of each of the three major communication systems in the host intestinal functions. This work reveals that P. aeruginosa influences intestinal inflammation and permeability through distinct mechanisms.
Keywords: LasR; MvfR; PqsR; Pseudomonas aeruginosa; RhlR; antivirulence; intestinal inflammation; intestinal permeability; quorum sensing; virulence.
Conflict of interest statement
The authors declare a conflict of interest. L.G.R. has a financial interest in Spero Therapeutics, a company developing therapies to treat bacterial infections. L.G.R.’s financial interests are reviewed and managed by Massachusetts General Hospital and Partners Health Care in accordance with their conflict-of-interest policies. No funding was received from Spero Therapeutics and had no role in study design, data collection, analysis, interpretation, or the decision to submit the work for publication. The rest of the authors declare no financial interest in Spero Therapeutics.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
