Positron Emission Tomography-Based Pharmacokinetic Analysis To Assess Renal Transporter-Mediated Drug-Drug Interactions of Antimicrobial Drugs
- PMID: 36786609
- PMCID: PMC10019293
- DOI: 10.1128/aac.01493-22
Positron Emission Tomography-Based Pharmacokinetic Analysis To Assess Renal Transporter-Mediated Drug-Drug Interactions of Antimicrobial Drugs
Abstract
Transporter-mediated drug-drug interactions (DDIs) are of concern in antimicrobial drug development, as they can have serious safety consequences. We used positron emission tomography (PET) imaging-based pharmacokinetic (PK) analysis to assess the effect of different drugs, which may cause transporter-mediated DDIs, on the tissue distribution and excretion of [18F]ciprofloxacin as a radiolabeled model antimicrobial drug. Mice underwent PET scans after intravenous injection of [18F]ciprofloxacin, without and with pretreatment with either probenecid (150 mg/kg), cimetidine (50 mg/kg), or pyrimethamine (5 mg/kg). A 3-compartment kidney PK model was used to assess the involvement of renal transporters in the examined DDIs. Pretreatment with probenecid and cimetidine significantly decreased the renal clearance (CLrenal) of [18F]ciprofloxacin. The effect of cimetidine (-86%) was greater than that of probenecid (-63%), which contrasted with previously published clinical data. The kidney PK model revealed that the decrease in CLrenal was caused by inhibition of basal uptake transporters and apical efflux transporters in kidney proximal tubule cells. Changes in the urinary excretion of [18F]ciprofloxacin after pretreatment with probenecid and cimetidine resulted in increased blood and organ exposure to [18F]ciprofloxacin. Our results suggest that multiple membrane transporters mediate the tubular secretion of ciprofloxacin, with possible species differences between mice and humans. Concomitant medication inhibiting renal transporters may precipitate DDIs, leading to decreased urinary excretion and increased blood and organ exposure to ciprofloxacin, potentially exacerbating adverse effects. Our study highlights the strength of PET imaging-based PK analysis to assess transporter-mediated DDIs at a whole-body level.
Keywords: antimicrobial drug disposition; drug-drug interaction; membrane transporters; positron emission tomography; renal clearance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
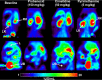

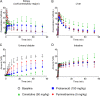

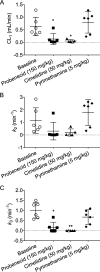
References
-
- Giacomini KM, Huang S-M, Tweedie DJ, Benet LZ, Brouwer KLR, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L, International Transporter Consortium . 2010. Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. 10.1038/nrd3028. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

