Endogenous IL-33 Accelerates Metacestode Growth during Late-Stage Alveolar Echinococcosis
- PMID: 36786637
- PMCID: PMC10101030
- DOI: 10.1128/spectrum.04239-22
Endogenous IL-33 Accelerates Metacestode Growth during Late-Stage Alveolar Echinococcosis
Abstract
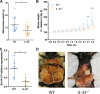
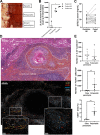
During the course of the infectious disease alveolar echinococcosis (AE), the larval stage of Echinococcus multilocularis develops in the liver, where an initial Th1/Th17 immune response may allow its elimination in resistant individuals. In patients susceptible to infection and disease, the Th2 response initiates later, inducing tolerance to the parasite. The role of interleukin 33 (IL-33), an alarmin released during necrosis and known to drive a Th2 immune response, has not yet been described during AE. Wild-type (WT) and IL-33-/- C57BL/6J mice were infected by peritoneal inoculation with E. multilocularis metacestodes and euthanized 4 months later, and their immune response were analyzed. Immunofluorescence staining and IL-33 enzyme-linked immunosorbent assay (ELISA) were also performed on liver samples from human patients with AE. Overall, metacestode lesions were smaller in IL-33-/- mice than in WT mice. IL-33 was detected in periparasitic tissues, but not in mouse or human serum. In infected mice, endogenous IL-33 modified peritoneal macrophage polarization and cytokine profiles. Th2 cytokine concentrations were positively correlated with parasite mass in WT mice, but not in IL-33-/- mice. In human AE patients, IL-33 concentrations were higher in parasitic tissues than in distant liver parenchyma. The main sources of IL-33 were CD31+ endothelial cells of the neovasculature, present within lymphoid periparasitic infiltrates together with FOXP3+ Tregs. In the murine model, periparasitic IL-33 correlated with accelerated parasite growth putatively through the polarization of M2-like macrophages and release of immunosuppressive cytokines IL-10 and transforming growth factor β1 (TGF-β1). We concluded that IL-33 is a key alarmin in AE that contributes to the tolerogenic effect of systemic Th2 cytokines. IMPORTANCE Infection with the metacestode stage of Echinococcus multilocularis, known as alveolar echinococcosis, is the most severe cestodosis worldwide. However, less than 1% of exposed individuals, in which the immune system is unable to control the parasite, develop the disease. The factors responsible for this interindividual variability are not fully understood. In this in vivo study comparing wild-type and IL-33-/- infected mice, together with data from human clinical samples, we determined that IL-33, an alarmin released following tissue injury and involved in the pathogenesis of cancer and asthma, accelerates the progression of the disease by modulating the periparasitic microenvironment. This suggests that targeting IL-33 could be of interest for the management of patients with AE, and that IL-33 polymorphisms could be responsible for increased susceptibility to AE.
Keywords: Echinococcus multilocularis; IL-1RL1; IL-33; ST2; alveolar echinococcosis; angiogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Vuitton DA, McManus DP, Rogan MT, Romig T, Gottstein B, Naidich A, Tuxun T, Wen H, da Silva AM, Vuitton DA, McManus DP, Romig T, Rogan MR, Gottstein B, da Silva AM, Wen H, Naidich A, Tuxun T, Avcioglu A, Boufana B, Budke C, Casulli A, Güven E, Hillenbrand A, Jalousian F, Jemli MH, Knapp J, Laatamna A, Lahmar S, Naidich A, Rogan MT, Sadjjadi SM, Schmidberger J, Amri M, Bellanger A-P, Benazzouz S, Brehm K, Hillenbrand A, Jalousian F, Kachani M, Labsi M, Masala G, da Silva AM, Seyed MS, Soufli I, Touil-Boukoffa C, Wang J, Zeyhle E, Aji T, Akhan O, World Association of Echinococcosis, et al. . 2020. International consensus on terminology to be used in the field of echinococcoses. Parasite 27:41. doi:10.1051/parasite/2020024. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

