Hydrophobic Ion Pairing of Small Molecules: How to Minimize Premature Drug Release from SEDDS and Reach the Absorption Membrane in Intact Form
- PMID: 36786693
- PMCID: PMC10015432
- DOI: 10.1021/acsbiomaterials.2c01504
Hydrophobic Ion Pairing of Small Molecules: How to Minimize Premature Drug Release from SEDDS and Reach the Absorption Membrane in Intact Form
Abstract
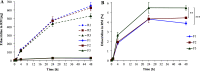
The present work aimed to form hydrophobic ion pairs (HIPs) of a small molecule remaining inside the oily droplets of SEDDS to a high extent. HIPs of ethacridine and various surfactants classified by functional groups of phosphates, sulfates, and sulfonates were formed and precipitation efficiency, log Dn-octanol/water, and solubility in different excipients were investigated. Most lipophilic HIPs were incorporated into SEDDS and evaluated regarding drug release. Docusate HIPs showed the highest increase in lipophilicity with a precipitation efficiency of 100%, a log Dn-octanol/water of 2.66 and a solubility of 132 mg/mL in n-octanol, 123 mg/mL in oleyl alcohol, and 40 mg/mL in medium chain triglycerides. Docusate HIPs were incorporated into three SEDDS of increasing lipophilicity (F1 < F2 < F3) based on medium chain triglycerides, oleyl alcohol, Kolliphor EL, and Tween 80 (F1: 1 + 5 + 2 + 2; F2: 3 + 3 + 2 + 2; F3: 5 + 1 + 4 + 0). Highest achievable payloads ranged from 74.49 mg/mL (F3) to 97.13 mg/mL (F1) and log DSEDDS/RM increased by at least 7.5 units (4.99, F1). Drug release studies via the diffusion membrane method confirmed minor release of docusate HIPs from all SEDDS (<2.7% within 4 h). In conclusion, highly lipophilic HIPs remain inside the oily phase of SEDDS and likely reach the absorption membrane in intact form.
Keywords: drug release; ethacridine; hydrophobic ion pairing; oral drug delivery; self-emulsifying drug delivery systems; small molecule.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

