Discriminative Accuracy of the CAPTURE Tool for Identifying Chronic Obstructive Pulmonary Disease in US Primary Care Settings
- PMID: 36786790
- PMCID: PMC9929696
- DOI: 10.1001/jama.2023.0128
Discriminative Accuracy of the CAPTURE Tool for Identifying Chronic Obstructive Pulmonary Disease in US Primary Care Settings
Abstract
Importance: Chronic obstructive pulmonary disease (COPD) is underdiagnosed in primary care.
Objective: To evaluate the operating characteristics of the CAPTURE (COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk) screening tool for identifying US primary care patients with undiagnosed, clinically significant COPD.
Design, setting, and participants: In this cross-sectional study, 4679 primary care patients aged 45 years to 80 years without a prior COPD diagnosis were enrolled by 7 primary care practice-based research networks across the US between October 12, 2018, and April 1, 2022. The CAPTURE questionnaire responses, peak expiratory flow rate, COPD Assessment Test scores, history of acute respiratory illnesses, demographics, and spirometry results were collected.
Exposure: Undiagnosed COPD.
Main outcomes and measures: The primary outcome was the CAPTURE tool's sensitivity and specificity for identifying patients with undiagnosed, clinically significant COPD. The secondary outcomes included the analyses of varying thresholds for defining a positive screening result for clinically significant COPD. A positive screening result was defined as (1) a CAPTURE questionnaire score of 5 or 6 or (2) a questionnaire score of 2, 3, or 4 together with a peak expiratory flow rate of less than 250 L/min for females or less than 350 L/min for males. Clinically significant COPD was defined as spirometry-defined COPD (postbronchodilator ratio of forced expiratory volume in the first second of expiration [FEV1] to forced vital capacity [FEV1:FVC] <0.70 or prebronchodilator FEV1:FVC <0.65 if postbronchodilator spirometry was not completed) combined with either an FEV1 less than 60% of the predicted value or a self-reported history of an acute respiratory illness within the past 12 months.
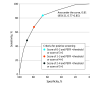
Results: Of the 4325 patients who had adequate data for analysis (63.0% were women; the mean age was 61.6 years [SD, 9.1 years]), 44.6% had ever smoked cigarettes, 18.3% reported a prior asthma diagnosis or use of inhaled respiratory medications, 13.2% currently smoked cigarettes, and 10.0% reported at least 1 cardiovascular comorbidity. Among the 110 patients (2.5% of 4325) with undiagnosed, clinically significant COPD, 53 had a positive screening result with a sensitivity of 48.2% (95% CI, 38.6%-57.9%) and a specificity of 88.6% (95% CI, 87.6%-89.6%). The area under the receiver operating curve for varying positive screening thresholds was 0.81 (95% CI, 0.77-0.85).
Conclusions and relevance: Within this US primary care population, the CAPTURE screening tool had a low sensitivity but a high specificity for identifying clinically significant COPD defined by presence of airflow obstruction that is of moderate severity or accompanied by a history of acute respiratory illness. Further research is needed to optimize performance of the screening tool and to understand whether its use affects clinical outcomes.
Conflict of interest statement
Figures

Comment in
-
Discriminative Accuracy of the CAPTURE Tool for Identifying COPD.JAMA. 2023 Jun 13;329(22):1986-1987. doi: 10.1001/jama.2023.7316. JAMA. 2023. PMID: 37314280 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

