Elucidating the Mechanisms of Dynamic and Robust Control of the Liver Homeostatic Renewal Process: Cell Network Modeling and Analysis
- PMID: 36787103
- PMCID: PMC9912253
- DOI: 10.1021/acs.iecr.2c03579
Elucidating the Mechanisms of Dynamic and Robust Control of the Liver Homeostatic Renewal Process: Cell Network Modeling and Analysis
Abstract
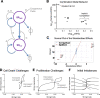
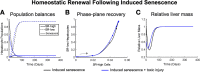
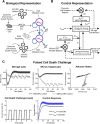
Recent experimental investigations of liver homeostatic renewal have identified high replication capacity hepatocyte populations as the primary maintainers of liver mass. However, the molecular and cellular processes controlling liver homeostatic renewal remain unknown. To address this problem, we developed and analyzed a mathematical model describing cellular network interactions underlying liver homeostatic renewal. Model simulation results demonstrate that without feedback control, basic homeostatic renewal is not robust to disruptions, leading to tissue loss under persistent/repetitive insults. Consequently, we extended our basic model to incorporate putative regulatory interactions and investigated how such interactions may confer robustness on the homeostatic renewal process. We utilized a Design of Experiments approach to identify the combination of feedback interactions that yields a cell network model of homeostatic renewal capable of maintaining liver mass robustly during persistent/repetitive injury. Simulations of this robust model indicate that repeated injury destabilizes liver homeostasis within several months, which differs from epidemiological observations of a much slower decay of liver function occurring over several years. To address this discrepancy, we extended the model to include feedback control by liver nonparenchymal cells. Simulations and analysis of the final multicellular feedback control network suggest that achieving robust liver homeostatic renewal requires intrinsic stability in a hepatocellular network combined with feedback control by nonparenchymal cells.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Diploid hepatocytes drive physiological liver renewal in adult humans.Cell Syst. 2022 Jun 15;13(6):499-507.e12. doi: 10.1016/j.cels.2022.05.001. Epub 2022 May 31. Cell Syst. 2022. PMID: 35649419
-
Design principles for the analysis and construction of robustly homeostatic biological networks.J Theor Biol. 2016 Nov 7;408:274-289. doi: 10.1016/j.jtbi.2016.06.036. Epub 2016 Jul 1. J Theor Biol. 2016. PMID: 27378006
-
Robust dynamic balance of AP-1 transcription factors in a neuronal gene regulatory network.BMC Syst Biol. 2010 Dec 17;4:171. doi: 10.1186/1752-0509-4-171. BMC Syst Biol. 2010. PMID: 21167049 Free PMC article.
-
The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal.Genes (Basel). 2020 Sep 30;11(10):1163. doi: 10.3390/genes11101163. Genes (Basel). 2020. PMID: 33008122 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
References
-
- van Es J. H.; Haegebarth A.; Kujala P.; Itzkovitz S.; Koo B.-K.; Boj S. F.; Korving J.; van den Born M.; van Oudenaarden A.; Robine S.; Clevers H. A Critical Role for the Wnt Effector Tcf4 in Adult Intestinal Homeostatic Self-Renewal. Mol. Cell. Biol. 2012, 32 (10), 1918–1927. 10.1128/MCB.06288-11. - DOI - PMC - PubMed
-
- Sousa-Victor P.; Gutarra S.; García-Prat L.; Rodriguez-Ubreva J.; Ortet L.; Ruiz-Bonilla V.; Jardí M.; Ballestar E.; González S.; Serrano A. L.; Perdiguero E.; Muñoz-Cánoves P. Geriatric Muscle Stem Cells Switch Reversible Quiescence into Senescence. Nature 2014, 506 (7488), 316–321. 10.1038/nature13013. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
