Early-life peripheral infections reprogram retinal microglia and aggravate neovascular age-related macular degeneration in later life
- PMID: 36787231
- PMCID: PMC9927938
- DOI: 10.1172/JCI159757
Early-life peripheral infections reprogram retinal microglia and aggravate neovascular age-related macular degeneration in later life
Abstract
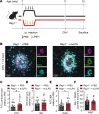
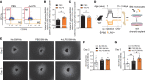
Pathological neovascularization in age-related macular degeneration (nvAMD) drives the principal cause of blindness in the elderly. While there is a robust genetic association between genes of innate immunity and AMD, genome-to-phenome relationships are low, suggesting a critical contribution of environmental triggers of disease. Possible insight comes from the observation that a past history of infection with pathogens such as Chlamydia pneumoniae, or other systemic inflammation, can predispose to nvAMD in later life. Using a mouse model of nvAMD with prior C. pneumoniae infection, endotoxin exposure, and genetic ablation of distinct immune cell populations, we demonstrated that peripheral infections elicited epigenetic reprogramming that led to a persistent memory state in retinal CX3CR1+ mononuclear phagocytes (MNPs). The immune imprinting persisted long after the initial inflammation had subsided and ultimately exacerbated choroidal neovascularization in a model of nvAMD. Single-cell assay for transposase-accessible chromatin sequencing (scATAC-seq) identified activating transcription factor 3 (ATF3) as a central mediator of retina-resident MNP reprogramming following peripheral inflammation. ATF3 polarized MNPs toward a reparative phenotype biased toward production of proangiogenic factors in response to subsequent injury. Therefore, a past history of bacterial endotoxin-induced inflammation can lead to immunological reprograming within CNS-resident MNPs and aggravate pathological angiogenesis in the aging retina.
Keywords: Cellular immune response; Endothelial cells; Ophthalmology.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

