Mitochondrial function in macrophages controls cardiac repair after myocardial infarction
- PMID: 36787252
- PMCID: PMC9927924
- DOI: 10.1172/JCI167079
Mitochondrial function in macrophages controls cardiac repair after myocardial infarction
Abstract
Cardiac healing following acute myocardial infarction (MI) involves the mobilization and activation of immune cells, including macrophages. In the early phase after MI, macrophages adopt a proinflammatory phenotype, while polarizing toward a reparative one in the late stage. Although metabolic reprogramming has been observed during this transition, the mechanistic links to macrophage differentiation are still poorly understood. In this issue of the JCI, Cai, Zhao and colleagues demonstrate that mitochondrial function in macrophages governed the resolution of inflammation and tissue repair by modulating the phagocytic removal of apoptotic cells (so-called efferocytosis) as well as myofibroblast activation. These findings provide important mechanistic insights into the potential relevance of metabolic modulation of macrophage functions following MI, which might lead to alternative therapeutic strategies for MI.
Conflict of interest statement
Figures
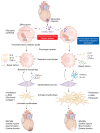
Comment on
- Mitochondrial Dysfunction in Macrophages Promotes Inflammation and Suppresses Repair After Myocardial Infarction

