Analysis of the clinical features and risk factors of device-related pressure injuries in the operating room
- PMID: 36787265
- PMCID: PMC9927895
- DOI: 10.1111/iwj.13912
Analysis of the clinical features and risk factors of device-related pressure injuries in the operating room
Abstract
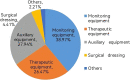
To describe the clinical features and risk factors of device-related pressure injuries (DRPIs) in the operating room. The clinical features of the DRPIs in patients undergoing elective surgery in a tertiary hospital in 2020 were investigated through prospective data collection. A DRPI-related questionnaire was designed for the patients, and those who did not experience any DRPI were selected according to a ratio of 1:2. Logistic regression analysis was performed in terms of the independent risk factors of operating-room DRPIs. A P-value of <.05 indicated a statistically significant difference. The incidence of operating-room DRPIs was 0.56%, and the proportion of stage I injuries was 73.53%. The injury-related devices included vital monitoring devices (31.62%), auxiliary therapy devices (27.94%), therapy devices (19.12%), and dressings (3.67%). Non-bone protuberances, such as the upper arms and thighs, were common injury sites. The patients' body mass index, mean arterial pressure, and instrument action time were independent risk factors for the operating-room DRPIs. To reduce the incidence of operating-room DRPIs, it is of great clinical significance to focus on the characteristics of the surgical patients and the types of surgery-related devices used and to take personalised preventive measures based on the relevant risk factors.
Keywords: clinical features; device-related injury; operating room; pressure injury; risk factors.
© 2022 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
Similar articles
-
Incidence, prevalence and risk factors of device-related pressure injuries in adult intensive care unit: A meta-analysis of 10,084 patients from 11 countries.Wound Repair Regen. 2023 Sep-Oct;31(5):713-722. doi: 10.1111/wrr.13112. Epub 2023 Aug 28. Wound Repair Regen. 2023. PMID: 37587087 Review.
-
Medical device-related pressure injuries in the operating room: A scoping review.J Adv Nurs. 2025 Mar;81(3):1208-1221. doi: 10.1111/jan.16400. Epub 2024 Aug 20. J Adv Nurs. 2025. PMID: 39164036
-
Device-related pressure injuries in adult intensive care unit patients: An Australian and New Zealand point prevalence study.Aust Crit Care. 2021 Nov;34(6):561-568. doi: 10.1016/j.aucc.2020.12.011. Epub 2021 Feb 20. Aust Crit Care. 2021. PMID: 33622521
-
Prevention of Pressure Injuries in the Operating Room: A Quality Improvement Project.J Wound Ostomy Continence Nurs. 2018 Mar/Apr;45(2):141-145. doi: 10.1097/WON.0000000000000410. J Wound Ostomy Continence Nurs. 2018. PMID: 29521925
-
Risk factors for device-related pressure injuries in general ward inpatients of a tertiary general hospital: A case-control study.J Tissue Viability. 2023 Nov;32(4):601-606. doi: 10.1016/j.jtv.2023.07.006. Epub 2023 Jul 29. J Tissue Viability. 2023. PMID: 37558560
Cited by
-
The Incidence of Pressure Ulcers in Surgical Patients: A Systematic Review.Int Wound J. 2025 Aug;22(8):e70738. doi: 10.1111/iwj.70738. Int Wound J. 2025. PMID: 40825923 Free PMC article. Review.
-
Explore the effect of pressure and time of compression on the risk of intraoperatively acquired pressure injury based on theoretical framework: A prospective study.Int Wound J. 2024 Apr;21(4):e14809. doi: 10.1111/iwj.14809. Int Wound J. 2024. PMID: 38613408 Free PMC article.
-
Pressure ulcer risk in patients undergoing cardiovascular surgery and their occurrence within 24 hours of the operation.Rev Esc Enferm USP. 2025 Aug 4;59:e20250081. doi: 10.1590/1980-220X-REEUSP-2025-0081en. eCollection 2025. Rev Esc Enferm USP. 2025. PMID: 40762991 Free PMC article.
-
Factors influencing the severity of medical device-related pressure injuries: Pressure injury staging comparison.Int Wound J. 2023 Sep;20(7):2735-2741. doi: 10.1111/iwj.14147. Epub 2023 Mar 20. Int Wound J. 2023. PMID: 36938762 Free PMC article.
References
-
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance , Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. Clinical practice guideline[S]. EPUAP/NPIAP/PPPIA; 2019. https://www.internationalguideline.com/
MeSH terms
LinkOut - more resources
Full Text Sources
Medical