Clinic-based SAMBA-II vs centralized laboratory viral load assays among HIV-1 infected children, adolescents and young adults in rural Zimbabwe: A randomized controlled trial
- PMID: 36787296
- PMCID: PMC9928130
- DOI: 10.1371/journal.pone.0281279
Clinic-based SAMBA-II vs centralized laboratory viral load assays among HIV-1 infected children, adolescents and young adults in rural Zimbabwe: A randomized controlled trial
Abstract
Background: In Zimbabwe, children, adolescents and young adults living with HIV (CALWH) who are on public health antiretroviral therapy (ART) have inadequate viral load (VL) suppression. We assessed whether a clinic-based VL monitoring could decrease 12-month virologic failure rates among these CALWH.
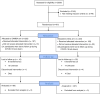
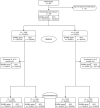
Methods: The study was registered on ClinicalTrials.gov: NCT03986099. CALWH in care at Chidamoyo Christian Hospital (CCH) and 8 rural outreach sites (ROS) on long-term community-based ART were randomized (1:1) to 6 monthly VL monitoring by COBAS®Ampliprep®/Taqman48® HIV-1 at the provincial referral laboratory (PRL) as per standard of care (SOC) or by the clinic-based SAMBA II assay, Diagnostics for the Real World, at CCH. VL suppression, turn-around-time (TAT) for VL results, drug switching and drug resistance in second-line failure were assessed at 12 months.
Results: Of 390 CALWH enrolled 347 (89%) completed 12 months follow-up. Median (IQR) age and ART duration were 14.1 (9.7-18.2) and 6.4 (3.7-7.9) years, respectively. Over half (57%) of the participants were female. At enrolment, 78 (20%) had VL ≥1,000 copies/ml and VL suppression of 80% was unchanged after 12 months, with no significant difference between the SOC (81%) and the clinic-based (80%) arms (p = 0.528). Median (IQR) months to confirmatory VL result at CCH vs PRL was 4.0 (2.1-4.4) vs 4.5 (3.5-6.3) respectively; p = 0.027 at 12 months. Drug switching was documented among 26/347 (7%) participants with no difference between the median (IQR) time to switch in SOC vs clinic-based arms (5.1 (3.9-10.0) months vs 4.4 (2.5-8.4) respectively; p = 0.569). Out of 24 confirmed second-line failures, only 4/19 (21%) had protease inhibitor resistance.
Conclusion: In rural Zimbabwe, the clinic-based SAMBA II assay was able to provide confirmatory VL results faster than the SOC VL assay at the PRL. However, this rapid TAT did not allow for a more efficient drug switch among these CALWH.
Copyright: © 2023 Kouamou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
At the time of the study, DI and MB were paid employees of Gilead Sciences Inc. There are no patents, products in development or marketed products to declare. This does not alter our adherence to policies on sharing data and materials.
Figures
References
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; 2016. - PubMed
-
- Granich R, Gupta S, Wollmers M, Ruffner M, Williams B. Modeling the HIV epidemic: why the 95-95-95Target and ART effectiveness parameters matter. Int J Virol AIDS. 2018;5(1).
-
- Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. Int Health. 2013;5(3):169–79. doi: 10.1093/inthealth/iht016 - DOI - PubMed
-
- Joint United Nations Programme. 2020 Global AIDS Update —Seizing the moment —Tackling entrenched inequalities to end epidemics. [Internet]. 2020. [cited 2020 Jul 7]. Available from: https://www.unaids.org/en/resources/documents/2020/global-aids-report