Considerations for the Terminal Sterilization of Oligonucleotide Drug Products
- PMID: 36787481
- PMCID: PMC10277985
- DOI: 10.1089/nat.2022.0073
Considerations for the Terminal Sterilization of Oligonucleotide Drug Products
Abstract
A primary function of the parenteral drug product manufacturing process is to ensure sterility of the final product. The two most common methods for sterilizing parenteral drug products are terminal sterilization (TS), whereby the drug product is sterilized in the final container following filling and finish, and membrane sterilization, whereby the product stream is sterilized by membrane filtration and filled into presterilized containers in an aseptic processing environment. Although TS provides greater sterility assurance than membrane sterilization and aseptic processing, not all drug products are amenable to TS processes, which typically involve heat treatment or exposure to ionizing radiation. Oligonucleotides represent an emerging class of therapeutics with great potential for treating a broad range of indications, including previously undruggable targets. Owing to their size, structural complexity, and relative lack of governing regulations, several challenges in drug development are unique to oligonucleotides. This exceptionality justifies a focused assessment of traditional chemistry, manufacturing, and control strategies before their adoption. In this article, we review the current state of sterile oligonucleotide drug product processing, highlight the key aspects to consider when assessing options for product sterilization, and provide recommendations to aid in the successful evaluation and development of TS processes. We also explore current regulatory expectations and provide our interpretation as it pertains to oligonucleotide drug products.
Keywords: autoclave; degradation; drug product; formulation; oligonucleotide; terminal sterilization.
Conflict of interest statement
J.S. and J.F. are employed by Ionis Pharmaceuticals, Inc.; D.D. is employed by Amgen and previously at Biogen; A.T., N.A., and D.R. are employed by AstraZeneca; C.L. and N.A. are employed by Novartis; M.F. and S.P. are employed by GlaxoSmithKline; A.W. is employed by Merck; G.G. is employed by Biogen; P.L.S is employed by Pfizer; K.S. is employed by F. Hoffmann-La Roche Ltd. No competing financial interests exist.
Figures





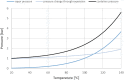
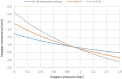
References
-
- Tivesten A, Akhtar N, Stolee J, Fillon Y, Orr R, Hill ML, Van den Bergh L, Huybrechts T, Muslehiddinoglu J and P McCormac. (2018). European Pharma Oligonucleotide Consortium: a move to consolidate oligonucleotide knowledge and share experience within the community. Ther Innov Regul Sci 52:687–688. - PubMed
-
- EMA. (2019). Guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. EMA/CHMP/CVMP/QWP/BWP/850374/2015. European Medicines Agency. www.ema.europa.eu/en/sterilisation-medicinal-product-active-substance-ex...
-
- PMDA. (2012). Guidance on the Manufacture of Sterile Pharmaceutical Products Produced by Terminal Sterilisation. www.pmda.go.jp/files/000160794.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

