Clonal expansion of resident memory T cells in peripheral blood of patients with non-small cell lung cancer during immune checkpoint inhibitor treatment
- PMID: 36787939
- PMCID: PMC9930609
- DOI: 10.1136/jitc-2022-005509
Clonal expansion of resident memory T cells in peripheral blood of patients with non-small cell lung cancer during immune checkpoint inhibitor treatment
Abstract
Background: Immune checkpoint inhibitors (ICIs) are an essential treatment for non-small cell lung cancer (NSCLC). Currently, the tumor-related intrinsic factors in response to ICIs have mostly been elucidated in tissue samples. However, tissue immune status and changes in the immune microenvironment can also be reflected and monitored through peripheral blood.
Methods: Single-cell RNA and T cell receptor (scTCR) sequencing were conducted using peripheral blood mononuclear cells (PBMCs) from 60 patients with stage IV NSCLC. Those samples were prospectively acquired from patients treated with anti-PD(L)-1 therapy for advanced lung cancer. Based on the clinical outcomes, samples were classified as durable clinical benefit (DCB) and non-durable clinical benefit (NCB). The samples constituted paired longitudinal samples, consisting of pre-treatment and on-treatment. Additionally, PBMC samples from 60 healthy donors from the Asian Immune Diversity Atlas project were used as a control.
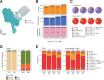
Results: The dynamic changes in major cell types between pre-treatment and on-treatment PBMCs were associated with an increase in proliferating T cells and NK cells in both DCB and NCB groups. Among T cell subtypes, effector memory CD8+ T cells (CD8+ TEM_GZMK_PDCD1) were increased after ICI treatment in both DCB and NCB. From the lineage trajectory analysis, effector memory CD8+ T cells resided at the bifurcation point, which has the potential to differentiate into lineages with precursor exhausted CD8+ T cells (CD8+ TCM cells) assumed to be related to the ICI response. From the scTCR-seq, effector memory CD8+ T cells along with T cells recognizing unknown antigen expanded and composed of novel clones skewed toward dysfunctional status, especially in on-treatment samples of the DCB group. The extent of immunophenotype conversion capabilities of the TCR with effector memory CD8+ T cells showed remarkable variation in the on-treatment sample in the DCB group.
Conclusion: A transitioning T cell subtype identified in PBMCs might be related to the prolonged ICI response. From our study, expansion of effector memory CD8+ T cells with novel TCRs in PBMCs after ICI treatment could contribute to a better clinical outcome in patients with NSCLC. This proof-of-concept research strengthens the use of non-invasive PBMCs in studying systemic changes of immune reactions related to the ICI treatment.
Keywords: Adaptive Immunity; Immunotherapy; Programmed Cell Death 1 Receptor; T-Lymphocytes; Tumor Biomarkers.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: S-HL reports grants and personal fees from MSD, personal fees from Novartis, personal fees from AstraZeneca, personal fees from BMS, personal fees from Roche, outside the submitted work. W-YP is a founder and CEO of Geninus Inc. All remaining authors have declared no conflicts of interest.
Figures



References
-
- Paz-Ares L, Ciuleanu T-E, Cobo M, et al. . First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198–211. 10.1016/S1470-2045(20)30641-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
