On-demand male contraception via acute inhibition of soluble adenylyl cyclase
- PMID: 36788210
- PMCID: PMC9929232
- DOI: 10.1038/s41467-023-36119-6
On-demand male contraception via acute inhibition of soluble adenylyl cyclase
Abstract
Nearly half of all pregnancies are unintended; thus, existing family planning options are inadequate. For men, the only choices are condoms and vasectomy, and most current efforts to develop new contraceptives for men impact sperm development, meaning that contraception requires months of continuous pretreatment. Here, we provide proof-of-concept for an innovative strategy for on-demand contraception, where a man would take a birth control pill shortly before sex, only as needed. Soluble adenylyl cyclase (sAC) is essential for sperm motility and maturation. We show a single dose of a safe, acutely-acting sAC inhibitor with long residence time renders male mice temporarily infertile. Mice exhibit normal mating behavior, and full fertility returns the next day. These studies define sAC inhibitors as leads for on-demand contraceptives for men, and they provide in vivo proof-of-concept for previously untested paradigms in contraception; on-demand contraception after just a single dose and pharmacological contraception for men.
© 2023. The Author(s).
Conflict of interest statement
Drs. Buck and Levin have licensed the commercialization of a panel of monoclonal antibodies directed against sAC to Millipore. Drs. Meinke, Steegborn, Levin, and Buck are co-founders of Sacyl Pharmaceuticals, Inc. established to develop sAC inhibitors into on-demand contraceptives. M.B., C.S., J.B., L.R.L., and employees of the Tri-Institutional Therapeutics Discovery Institute (TDI) may benefit from the further development and licensure of molecules described herein. All other authors declare no competing interests.
Figures


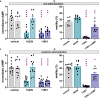

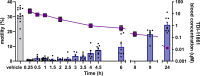
Comment in
-
Rapid reversible male birth-control treatment.Lab Anim (NY). 2023 May;52(5):101. doi: 10.1038/s41684-023-01174-5. Lab Anim (NY). 2023. PMID: 37137982 No abstract available.
References
-
- World Health Organization Task Force on methods for the regulation of male fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet336, 955–959 (1990). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

