Pulmonary recovery from COVID-19 in patients with metabolic diseases: a longitudinal prospective cohort study
- PMID: 36788324
- PMCID: PMC9926446
- DOI: 10.1038/s41598-023-29654-1
Pulmonary recovery from COVID-19 in patients with metabolic diseases: a longitudinal prospective cohort study
Abstract
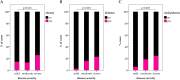
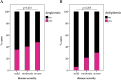
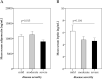
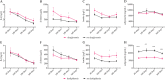
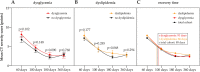
The severity of coronavirus disease 2019 (COVID-19) is related to the presence of comorbidities including metabolic diseases. We herein present data from the longitudinal prospective CovILD trial, and investigate the recovery from COVID-19 in individuals with dysglycemia and dyslipidemia. A total of 145 COVID-19 patients were prospectively followed and a comprehensive clinical, laboratory and imaging assessment was performed at 60, 100, 180, and 360 days after the onset of COVID-19. The severity of acute COVID-19 and outcome at early post-acute follow-up were significantly related to the presence of dysglycemia and dyslipidemia. Still, at long-term follow-up, metabolic disorders were not associated with an adverse pulmonary outcome, as reflected by a good recovery of structural lung abnormalities in both, patients with and without metabolic diseases. To conclude, dyslipidemia and dysglycemia are associated with a more severe course of acute COVID-19 as well as delayed early recovery but do not impair long-term pulmonary recovery.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

