The effect of combining therapeutic drug monitoring of antihypertensive drugs with personalised feedback on adherence and resistant hypertension: the (RHYME-RCT) trial protocol of a multi-centre randomised controlled trial
- PMID: 36788491
- PMCID: PMC9926861
- DOI: 10.1186/s12872-023-03114-0
The effect of combining therapeutic drug monitoring of antihypertensive drugs with personalised feedback on adherence and resistant hypertension: the (RHYME-RCT) trial protocol of a multi-centre randomised controlled trial
Abstract
Background: Adherence to antihypertensive drugs (AHDs) is important for adequate blood pressure control. Not taking these drugs as prescribed is one of the main underlying causes for resistant hypertension (RH), which in turn leads to an increased risk of cardiovascular events, stroke and kidney damage. Therefore, correct identification of patients that are non-adherent to AHDs is crucial to improve clinical outcome. For this goal, therapeutic drug monitoring is the most reliable method. The primary objective of this trial is to investigate whether monitoring of drug concentrations with a dried blood spot (DBS) sampling method combined with personalised feedback leads to a decrease in prevalence of RH after 12 months due to an increase in adherence. Secondary objectives include the difference over time in the number of required AHDs as well as the defined daily dose (DDD). Lastly, the cost-utility of SoC versus the intervention in RH is determined.
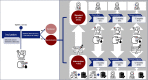
Methods: This is a multi-centre single-blinded randomised controlled trial (RHYME-RCT). First, at an eligibility visit, DBS sampling, to monitor drug concentrations in blood, and a 24-h ambulatory blood pressure measurement (24-h ABPM) are performed simultaneously. Patients with a daytime systolic blood pressure (SBP) > 135 and/or diastolic blood pressure (DBP) > 85 mmHg are randomised to SoC or intervention + SoC. The intervention is performed by the treating physician and includes information on drug concentrations and a comprehensive personalised feedback conversation with the use of a communication tool. The follow-up period is one year with visits at 3, 6 and 12 months randomisation and includes 24-h ABPM and DBS sampling.
Discussion: This will be the first trial that focusses specifically on patients with RH without taking into account suspicion of non-adherence and it combines monitoring of AHD concentrations to identify non-adherence to AHDs with a comprehensive feedback to improve non-adherence. Furthermore, if this trial shows positive outcomes for the intervention it can be directly implemented in clinical practice, which would be a great improvement in the treatment of RH.
Trial registration: RHYME-RCT is registered in the Dutch Trial Register on 27/12/2017 (NTR6914) and can be found in the International Clinical Trials Registry Platform.
Keywords: Adherence; Antihypertensive drugs; Blood pressure; Hypertension; Intervention; Therapeutic drug monitoring.
© 2023. The Author(s).
Conflict of interest statement
L.E.J. Peeters has received lecture fees from Astellas Pharma in 2021. T. van Gelder has received lecture fees and study grants from Chiesi and Astellas, in addition to consulting fees from Roche Diagnostics, Thermo Fisher, Vitaeris, CSL Behring, Astellas and Aurinia Pharma. In the last 3 years L. van Dijk has received a research grant from Teva for a study not related to this one. M.H.W. Kappers, E. Boersma, E.K. Massey, B.C.P. Koch and J. Versmissen have no conflicts of interest.
Figures
References
-
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. - DOI - PubMed
-
- De Geest S, Ruppar T, Berben L, Schonfeld S, Hill MN. Medication non-adherence as a critical factor in the management of presumed resistant hypertension: a narrative review. EuroInterven: J EuroPCR Collab Work Group Interven Cardiol Eur Soc Cardiol. 2014;9(9):1102–1109. doi: 10.4244/EIJV9I9A185. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials