A Potential Role of the Spike Protein in Neurodegenerative Diseases: A Narrative Review
- PMID: 36788995
- PMCID: PMC9922164
- DOI: 10.7759/cureus.34872
A Potential Role of the Spike Protein in Neurodegenerative Diseases: A Narrative Review
Abstract
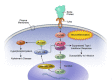
Human prion protein and prion-like protein misfolding are widely recognized as playing a causal role in many neurodegenerative diseases. Based on in vitro and in vivo experimental evidence relating to prion and prion-like disease, we extrapolate from the compelling evidence that the spike glycoprotein of SARS-CoV-2 contains extended amino acid sequences characteristic of a prion-like protein to infer its potential to cause neurodegenerative disease. We propose that vaccine-induced spike protein synthesis can facilitate the accumulation of toxic prion-like fibrils in neurons. We outline various pathways through which these proteins could be expected to distribute throughout the body. We review both cellular pathologies and the expression of disease that could become more frequent in those who have undergone mRNA vaccination. Specifically, we describe the spike protein's contributions, via its prion-like properties, to neuroinflammation and neurodegenerative diseases; to clotting disorders within the vasculature; to further disease risk due to suppressed prion protein regulation in the context of widely prevalent insulin resistance; and to other health complications. We explain why these prion-like characteristics are more relevant to vaccine-related mRNA-induced spike proteins than natural infection with SARS-CoV-2. We note with an optimism an apparent loss of prion-like properties among the current Omicron variants. We acknowledge that the chain of pathological events described throughout this paper is only hypothetical and not yet verified. We also acknowledge that the evidence we usher in, while grounded in the research literature, is currently largely circumstantial, not direct. Finally, we describe the implications of our findings for the general public, and we briefly discuss public health recommendations we feel need urgent consideration. An earlier version of this article was previously posted to the Authorea preprint server on August 16, 2022.
Keywords: amyloidosis; cd16+ monocytes; diabetes; exosomes; g quadruplexes; mrna vaccines; neurodegeneration; prion disease; sars-cov-2; spike protein.
Copyright © 2023, Seneff et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
