HMGB1 is a critical molecule in the pathogenesis of Gram-negative sepsis
- PMID: 36789020
- PMCID: PMC9924014
- DOI: 10.1016/j.jointm.2022.02.001
HMGB1 is a critical molecule in the pathogenesis of Gram-negative sepsis
Abstract
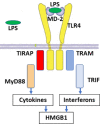
Gram-negative sepsis is a severe clinical syndrome associated with significant morbidity and mortality. Lipopolysaccharide (LPS), expressed on Gram-negative bacteria, is a potent pro-inflammatory toxin that induces inflammation and coagulation via two separate receptor systems. One is Toll-like receptor 4 (TLR4), expressed on cell surfaces and in endosomes, and the other is the cytosolic receptor caspase-11 (caspases-4 and -5 in humans). Extracellular LPS binds to high mobility group box 1 (HMGB1) protein, a cytokine-like molecule. The HMGB1-LPS complex is transported via receptor for advanced glycated end products (RAGE)-endocytosis to the endolysosomal system to reach the cytosolic LPS receptor caspase-11 to induce HMGB1 release, inflammation, and coagulation that may cause multi-organ failure. The insight that LPS needs HMGB1 assistance to generate severe inflammation has led to successful therapeutic results in preclinical Gram-negative sepsis studies targeting HMGB1. However, to date, no clinical studies have been performed based on this strategy. HMGB1 is also actively released by peripheral sensory nerves and this mechanism is fundamental for the initiation and propagation of inflammation during tissue injury. Homeostasis is achieved when other neurons actively restrict the inflammatory response via monitoring by the central nervous system and the vagus nerve through the cholinergic anti-inflammatory pathway. The neuronal control in Gram-negative sepsis needs further studies since a deeper understanding of the interplay between HMGB1 and acetylcholine may have beneficial therapeutic implications. Herein, we review the synergistic overlapping mechanisms of LPS and HMGB1 and discuss future treatment opportunities in Gram-negative sepsis.
Keywords: Caspase-11; High mobility group box 1 (HMGB1); Lipopolysaccharide (LPS); Receptor for advanced glycated end products (RAGE); Sepsis; Toll-like receptor 4 (TLR4).
© 2022 The Author(s). Published by Elsevier B.V. on behalf of Chinese Medical Association.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation.Mol Med. 2019 Apr 11;25(1):13. doi: 10.1186/s10020-019-0081-6. Mol Med. 2019. PMID: 30975096 Free PMC article.
-
High-mobility group box 1 inhibits HCO(3)(-) absorption in medullary thick ascending limb through a basolateral receptor for advanced glycation end products pathway.Am J Physiol Renal Physiol. 2015 Oct 15;309(8):F720-30. doi: 10.1152/ajprenal.00227.2015. Epub 2015 Jul 15. Am J Physiol Renal Physiol. 2015. PMID: 26180239 Free PMC article.
-
Lipopolysaccharide-Activated Canine Platelets Upregulate High Mobility Group Box-1 via Toll-Like Receptor 4.Front Vet Sci. 2021 Jun 21;8:674678. doi: 10.3389/fvets.2021.674678. eCollection 2021. Front Vet Sci. 2021. PMID: 34235204 Free PMC article.
-
Targeting HMGB1 for the treatment of sepsis and sepsis-induced organ injury.Acta Pharmacol Sin. 2022 Mar;43(3):520-528. doi: 10.1038/s41401-021-00676-7. Epub 2021 May 26. Acta Pharmacol Sin. 2022. PMID: 34040166 Free PMC article. Review.
-
A potential new pathway for heparin treatment of sepsis-induced lung injury: inhibition of pulmonary endothelial cell pyroptosis by blocking hMGB1-LPS-induced caspase-11 activation.Front Cell Infect Microbiol. 2022 Sep 15;12:984835. doi: 10.3389/fcimb.2022.984835. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189354 Free PMC article. Review.
Cited by
-
Commensal Bacteria Impact on Intestinal Toll-like Receptor Signaling in Salmonella-Challenged Gnotobiotic Piglets.Pathogens. 2023 Oct 29;12(11):1293. doi: 10.3390/pathogens12111293. Pathogens. 2023. PMID: 38003758 Free PMC article.
-
Optimal strategy for treatment of sepsis based on the host inflammatory reaction and immune response.J Intensive Med. 2023 Nov 18;4(2):175-180. doi: 10.1016/j.jointm.2023.10.002. eCollection 2024 Apr. J Intensive Med. 2023. PMID: 38681784 Free PMC article. No abstract available.
-
Preclinical development of the TLR4 antagonist FP12 as a drug lead targeting the HMGB1/MD-2/TLR4 axis in lethal influenza infection.Innate Immun. 2025 Jan-Dec;31:17534259241313201. doi: 10.1177/17534259241313201. Innate Immun. 2025. PMID: 40033742 Free PMC article.
-
Heparin in sepsis: current clinical findings and possible mechanisms.Front Immunol. 2024 Dec 6;15:1495260. doi: 10.3389/fimmu.2024.1495260. eCollection 2024. Front Immunol. 2024. PMID: 39712008 Free PMC article. Review.
-
Beyond the Toll-Like Receptor 4. Structure-Dependent Lipopolysaccharide Recognition Systems: How far are we?ChemMedChem. 2025 Mar 15;20(6):e202400780. doi: 10.1002/cmdc.202400780. Epub 2025 Jan 15. ChemMedChem. 2025. PMID: 39752323 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

