Low-molecular weight oligosaccharides from gum tragacanth (Astragalus gossypinus) ameliorate nonalcoholic fatty liver disease (NAFLD) in Wistar male rats
- PMID: 36789034
- PMCID: PMC9922153
- DOI: 10.1002/fsn3.3112
Low-molecular weight oligosaccharides from gum tragacanth (Astragalus gossypinus) ameliorate nonalcoholic fatty liver disease (NAFLD) in Wistar male rats
Erratum in
-
Erratum to "Low-molecular weight oligosaccharides from gum tragacanth (Astragalus gossypinus) ameliorate nonalcoholic fatty liver disease (NAFLD) in Wistar male rats".Food Sci Nutr. 2023 Apr 13;11(6):3616. doi: 10.1002/fsn3.3349. eCollection 2023 Jun. Food Sci Nutr. 2023. PMID: 37324911 Free PMC article.
Abstract
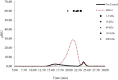
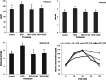
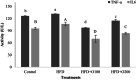
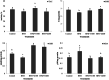
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease affecting 25% of the world's population. The effects of oligosaccharides from Gum tragacanth (Astragalus gossypinus) (GT) on oxidative stress, glucose metabolism, and expression of autophagy genes were investigated in induced non-alcoholic fatty liver. Twenty-four male healthy rats were divided into four groups, Control; high-fat diet, high-fat diet + 100 mg GT oligosaccharides/kg body weight, high-fat diet + 200 mg GT oligosaccharides/kg body weight and fed with the trial diets for 70 days. At the end of the experiment, the results indicated that GT oligosaccharides affected the weight gain and liver weight in NAFLD-induced rats. In addition, the results showed that the use of GT oligosaccharides significantly decreased oxidative stress, liver injury, and hyperglycemia (p < .05) and upregulated the expression of autophagy genes in NAFLD-induced rats.
Practical applications: Overall, the results of the current study demonstrated that the use of GT oligosaccharides obtained from Gum tragacanth (Astragalus gossypinus) showed significant antioxidant properties and hypoglycemia in NAFLD induced rats and could be used as a useful nutritional strategy for the prevention and treatment of NAFLD.
Keywords: autophagy; fatty liver; glycemia; gum tragacanth; oligosaccharides; oxidative stress.
© 2022 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
There is no conflict of interest in this paper.
Figures




Similar articles
-
Erratum to "Low-molecular weight oligosaccharides from gum tragacanth (Astragalus gossypinus) ameliorate nonalcoholic fatty liver disease (NAFLD) in Wistar male rats".Food Sci Nutr. 2023 Apr 13;11(6):3616. doi: 10.1002/fsn3.3349. eCollection 2023 Jun. Food Sci Nutr. 2023. PMID: 37324911 Free PMC article.
-
Effect of partial replacement of fat with added water and tragacanth gum (Astragalus gossypinus and Astragalus compactus) on the physicochemical, texture, oxidative stability, and sensory property of reduced fat emulsion type sausage.Meat Sci. 2019 Jan;147:135-143. doi: 10.1016/j.meatsci.2018.09.007. Epub 2018 Sep 10. Meat Sci. 2019. PMID: 30243231
-
Enzymatic depolymerization of gum tragacanth: bifidogenic potential of low molecular weight oligosaccharides.J Agric Food Chem. 2013 Feb 13;61(6):1272-8. doi: 10.1021/jf304795f. Epub 2013 Feb 4. J Agric Food Chem. 2013. PMID: 23343141
-
Prebiotic Xylo-Oligosaccharides Ameliorate High-Fat-Diet-Induced Hepatic Steatosis in Rats.Nutrients. 2020 Oct 22;12(11):3225. doi: 10.3390/nu12113225. Nutrients. 2020. PMID: 33105554 Free PMC article.
-
Protective effect of protein hydrolysates from Litopenaeus vannamei waste on oxidative status, glucose regulation, and autophagy genes in non-alcoholic fatty liver disease in Wistar rats.Iran J Basic Med Sci. 2022 Aug;25(8):954-963. doi: 10.22038/IJBMS.2022.62167.13761. Iran J Basic Med Sci. 2022. PMID: 36159326 Free PMC article.
Cited by
-
Mulberry and Hippophae-based solid beverage attenuate hyperlipidemia and hepatic steatosis via adipose tissue-liver axis.Food Sci Nutr. 2024 Apr 8;12(7):5052-5064. doi: 10.1002/fsn3.4155. eCollection 2024 Jul. Food Sci Nutr. 2024. PMID: 39055214 Free PMC article.
References
-
- Begriche, K. , Igoudjil, A. , Pessayre, D. , & Fromenty, B. (2006). Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion, 6, 1–28. - PubMed
-
- Bellentani, S. (2017). The epidemiology of non‐alcoholic fatty liver disease. Liver International, 37, 81–84. - PubMed
-
- Brunt, E. M. , Janney, C. G. , Di Bisceglie, A. M. , Neuschwander‐Tetri, B. A. , & Bacon, B. R. (1999). Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. The American Journal of Gastroenterology, 94, 2467–2474. - PubMed
-
- Carter‐Kent, C. , Zein, N. N. , & Feldstein, A. E. (2008). Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: Implications for treatment. Official Journal of the American College of Gastroenterology| ACG, 103, 1036–1042. - PubMed
-
- Chang, H.‐C. , Huang, C.‐N. , Yeh, D.‐M. , Wang, S.‐J. , Peng, C.‐H. , & Wang, C.‐J. (2013). Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods for Human Nutrition, 68, 18–23. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

