Inhibition of H1N1 by Picochlorum sp. 122 via AKT and p53 signaling pathways
- PMID: 36789072
- PMCID: PMC9922122
- DOI: 10.1002/fsn3.3110
Inhibition of H1N1 by Picochlorum sp. 122 via AKT and p53 signaling pathways
Abstract
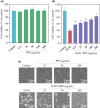
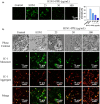
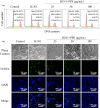
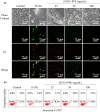
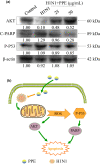
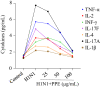
Influenza viruses cause a severe threat to global health, which can lead to annual epidemics and cause pandemics occasionally. However, the number of anti-influenza therapeutic agents is very limited. Polysaccharides, extracted from Picochlorum sp. (PPE), seaweed Polysaccharides, have exhibited antiviral activity and were expected to be used for influenza treatment. In our research, the capability of PPE to inhibit H1N1 infection was proved in MDCK cells. PPE could make MDCK cells avoid being infected with H1N1 and inhibited nuclear fragmentation and condensation of chromatin. PPE evidently inhibited the generation of reactive oxygen species in MDCK cells. Mechanism study revealed that PPE prevented MDCK cells from H1N1 infection through induction of apoptosis by stimulating AKT signaling pathway and suppressing p-p53 signaling pathway. In conclusion, PPE turns out to act as a prospective antiviral drug for H1N1 influenza.
Keywords: Polysaccharides; apoptosis; influenza virus; signaling pathway.
© 2022 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
The Inhibition of H1N1 Influenza Virus-Induced Apoptosis by Surface Decoration of Selenium Nanoparticles with β-Thujaplicin through Reactive Oxygen Species-Mediated AKT and p53 Signaling Pathways.ACS Omega. 2020 Nov 16;5(47):30633-30642. doi: 10.1021/acsomega.0c04624. eCollection 2020 Dec 1. ACS Omega. 2020. PMID: 33283112 Free PMC article.
-
Silver Nanoparticle Based Codelivery of Oseltamivir to Inhibit the Activity of the H1N1 Influenza Virus through ROS-Mediated Signaling Pathways.ACS Appl Mater Interfaces. 2016 Sep 21;8(37):24385-93. doi: 10.1021/acsami.6b06613. Epub 2016 Sep 12. ACS Appl Mater Interfaces. 2016. PMID: 27588566
-
Inhibition of enterovirus 71 infection by polysaccharides extracted from Picochlorum sp. 122 via the AKT and ATM/ATR signaling pathways.Arch Virol. 2021 Dec;166(12):3269-3274. doi: 10.1007/s00705-021-05229-1. Epub 2021 Sep 18. Arch Virol. 2021. PMID: 34536128
-
Non-hydrolyzed in digestive tract and blood natural L-carnosine peptide ("bioactivated Jewish penicillin") as a panacea of tomorrow for various flu ailments: signaling activity attenuating nitric oxide (NO) production, cytostasis, and NO-dependent inhibition of influenza virus replication in macrophages in the human body infected with the virulent swine influenza A (H1N1) virus.J Basic Clin Physiol Pharmacol. 2013;24(1):1-26. doi: 10.1515/jbcpp-2012-0037. J Basic Clin Physiol Pharmacol. 2013. PMID: 23425625 Review.
-
Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment.Molecules. 2024 May 17;29(10):2371. doi: 10.3390/molecules29102371. Molecules. 2024. PMID: 38792236 Free PMC article. Review.
Cited by
-
Selenium Nanoparticles Control H1N1 Virus by Inhibiting Inflammatory Response and Cell Apoptosis.Molecules. 2023 Aug 7;28(15):5920. doi: 10.3390/molecules28155920. Molecules. 2023. PMID: 37570890 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

