This is a preprint.
Macrophages play a leading role in determining the direction of astrocytic migration in spinal cord injury via ADP-P2Y1R axis
- PMID: 36789440
- PMCID: PMC9928047
- DOI: 10.21203/rs.3.rs-2427082/v1
Macrophages play a leading role in determining the direction of astrocytic migration in spinal cord injury via ADP-P2Y1R axis
Update in
-
Macrophages play a leading role in determining the direction of astrocytic migration in spinal cord injury via ADP-P2Y1R axis.Sci Rep. 2023 Jul 10;13(1):11177. doi: 10.1038/s41598-023-38301-8. Sci Rep. 2023. PMID: 37429920 Free PMC article.
Abstract
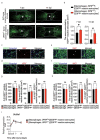
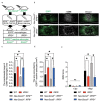
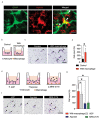
After spinal cord injury (SCI), inflammatory cells such as macrophages infiltrate the injured area, and astrocytes migrate, forming a glial scar around macrophages. The glial scar inhibits axonal regeneration, resulting in significant permanent disability. However, the mechanism by which glial scar-forming astrocytes migrate to the injury site has not been clarified. Here we show that migrating macrophages attract reactive astrocytes toward the center of the lesion after SCI. Chimeric mice with bone marrow lacking IRF8, which controls macrophage centripetal migration after SCI, showed widely scattered macrophages in injured spinal cord with the formation of a huge glial scar around the macrophages. To determine whether astrocytes or macrophages play a leading role in determining the directions of migration, we generated chimeric mice with reactive astrocyte-specific Socs3 -/- mice, which showed enhanced astrocyte migration, and bone marrow from IRF8 -/- mice. In this mouse model, macrophages were widely scattered, and a huge glial scar was formed around the macrophages as in wild-type mice that were transplanted with IRF8 -/ bone marrow. In addition, we revealed that macrophage-secreted ATP-derived ADP attracts astrocytes via the P2Y1 receptor. Our findings revealed a mechanism in which migrating macrophages attracted astrocytes and affected the pathophysiology and outcome after SCI.
Conflict of interest statement
Competing interests
The authors declare no competing interests in association with the present study.
Figures

References
-
- McDonald J. W. & Sadowsky C. Spinal-cord injury. Lancet 359, 417–425 (2002). - PubMed
-
- Kobayakawa K. et al. Macrophage centripetal migration drives spontaneous healing process after spinal cord injury. Sci. Adv vol. 5 https://www.science.org (2019). - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

