Variation in second breast cancer risk after primary invasive cancer by time since primary cancer diagnosis and estrogen receptor status
- PMID: 36789739
- PMCID: PMC10409444
- DOI: 10.1002/cncr.34679
Variation in second breast cancer risk after primary invasive cancer by time since primary cancer diagnosis and estrogen receptor status
Abstract
Background: In women with previously treated breast cancer, occurrence and timing of second breast cancers have implications for surveillance. The authors examined the timing of second breast cancers by primary cancer estrogen receptor (ER) status in the Breast Cancer Surveillance Consortium.
Methods: Women who were diagnosed with American Joint Commission on Cancer stage I-III breast cancer were identified within six Breast Cancer Surveillance Consortium registries from 2000 to 2017. Characteristics collected at primary breast cancer diagnosis included demographics, ER status, and treatment. Second breast cancer events included subsequent ipsilateral or contralateral breast cancers diagnosed >6 months after primary diagnosis. The authors examined cumulative incidence and second breast cancer rates by primary cancer ER status during 1-5 versus 6-10 years after diagnosis.
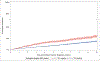
Results: At 10 years, the cumulative second breast cancer incidence was 11.8% (95% confidence interval [CI], 10.7%-13.1%) for women with ER-negative disease and 7.5% (95% CI, 7.0%-8.0%) for those with ER-positive disease. Women with ER-negative cancer had higher second breast cancer rates than those with ER-positive cancer during the first 5 years of follow-up (16.0 per 1000 person-years [PY]; 95% CI, 14.2-17.9 per 1000 PY; vs. 7.8 per 1000 PY; 95% CI, 7.3-8.4 per 1000 PY, respectively). After 5 years, second breast cancer rates were similar for women with ER-negative versus ER-positive breast cancer (12.1 per 1000 PY; 95% CI, 9.9-14.7; vs. 9.3 per 1000 PY; 95% CI, 8.4-10.3 per 1000 PY, respectively).
Conclusions: ER-negative primary breast cancers are associated with a higher risk of second breast cancers than ER-positive cancers during the first 5 years after diagnosis. Further study is needed to examine the potential benefit of more intensive surveillance targeting these women in the early postdiagnosis period.
Keywords: breast cancer; estrogen receptor; recurrence; surveillance; survivorship.
© 2023 American Cancer Society.
Conflict of interest statement
CONFLICTS OF INTEREST STATEMENT
The remaining authors made no disclosures.
Figures



References
-
- Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34(6):611–635. PubMed PMID: 26644543. doi:1200/JCO.2015.64.3809 - PubMed
-
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer, Version 5.2020. NCCN; 2020. Accessed July 21, 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
-
- Lewin AA, Moy L, et al. ACR Appropriateness Criteria® stage I breast cancer: initial workup and surveillance for local recurrence and distant metastases in asymptomatic women. J Am Coll Radiol. 2019;16(11S):S428–S439. Epub 2019/11/07PubMed PMID:31685110. doi:1016/j.jacr.2019.05.024 - PubMed
-
- Buist DSM, Ichikawa L, Wernli KJ, et al. Facility variability in examination indication among women with prior breast cancer: implications and the need for standardization. J Am Coll Radiol. 2020;17(6):755–764. Epub 2020/02/01PubMed PMID: 32004483; PubMed Central PMCID: PMCPMC7275918. doi:1016/j.jacr.2019. 12.020 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

