Pseudoprogression versus true progression in glioblastoma: what neurosurgeons need to know
- PMID: 36790010
- PMCID: PMC10412732
- DOI: 10.3171/2022.12.JNS222173
Pseudoprogression versus true progression in glioblastoma: what neurosurgeons need to know
Abstract
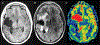
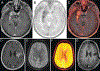
Management of patients with glioblastoma (GBM) is complex and involves implementing standard therapies including resection, radiation therapy, and chemotherapy, as well as novel immunotherapies and targeted small-molecule inhibitors through clinical trials and precision medicine approaches. As treatments have advanced, the radiological and clinical assessment of patients with GBM has become even more challenging and nuanced. Advances in spatial resolution and both anatomical and physiological information that can be derived from MRI have greatly improved the noninvasive assessment of GBM before, during, and after therapy. Identification of pseudoprogression (PsP), defined as changes concerning for tumor progression that are, in fact, transient and related to treatment response, is critical for successful patient management. These temporary changes can produce new clinical symptoms due to mass effect and edema. Differentiating this entity from true tumor progression is a major decision point in the patient's management and prognosis. Providers may choose to start an alternative therapy, transition to a clinical trial, consider repeat resection, or continue with the current therapy in hopes of resolution. In this review, the authors describe the invasive and noninvasive techniques neurosurgeons need to be aware of to identify PsP and facilitate surgical decision-making.
Keywords: glioblastoma; glioma; oncology; radiation necrosis; treatment-related effects; true progression; tumor; pseudoprogression.
Conflict of interest statement
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Figures




References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352(10):987–996. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

