Enterovirus A71 Promotes Exosome Secretion by the Nonstructural Protein 3A Interacting with Rab27a
- PMID: 36790212
- PMCID: PMC10101103
- DOI: 10.1128/spectrum.03446-22
Enterovirus A71 Promotes Exosome Secretion by the Nonstructural Protein 3A Interacting with Rab27a
Erratum in
-
Correction for Wu et al., "Enterovirus A71 Promotes Exosome Secretion by the Nonstructural Protein 3A Interacting with Rab27a".Microbiol Spectr. 2024 Oct 18;12(12):e0176624. doi: 10.1128/spectrum.01766-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 39422471 Free PMC article. No abstract available.
Abstract
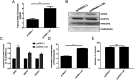
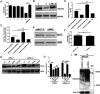
Exosomes are small membrane-bound vesicles which are intraluminal vesicles (ILVs) secreted to the extracellular space after multivesicular bodies (MVBs) fuse with the plasma membrane. Although it is known that exosomes play a multitude of roles during viral infection, the mechanism that regulates their secretion during viral infection is unknown. Here, we found that enterovirus A71 (EV-A71) infection increased exosome secretion both in vivo and in vitro. Importantly, the expression of nonstructural protein 3A was sufficient to promote exosome secretion, while a mutation affecting the amino acid 18 position abrogated this effect, without changing the size of exosomes in vivo or in vitro. Transmission electron microscopy (TEM) analysis revealed that 3A decreases the number of MVBs and ILVs in vivo and in vitro, which suggested 3A may boost the fusion between MVBs and the plasma membrane. Furthermore, we demonstrated that an interaction between 3A and the small GTPase protein, Rab27a, protected Rab27a from ubiquitination, resulted in increasing exosome release. Data indicated a novel mechanism by which EV-A71 3A modifies exosome secretion during viral infection. IMPORTANCE Research has shown that viral infection impacts exosome secretion, but its regulation mechanisms remain poorly understood. Nonstructural protein 3A of EV-A71 interacts with many host factors and is involved in the remodeling of cellular membranes. In this investigation, we applied exogenous expression of 3A protein for exploring its regulation on exosome secretion and utilized immunoprecipitation combined with proteomics approaches to identify 3A-interacting factors. Our results demonstrate that 3A protein upregulates the release of the exosomes and that the 3A mutant strain of EV-A71 induce less exosome release compared with the EV-A71 wild type. Viral 3A protein interacts with the host factor Rab27a to prevent it from being ubiquitinated, which in turn improves exosome secretion both in vitro and in vivo. EV-A71 3A protein is a novel viral factor in the control of exosome production.
Keywords: 3A; Rab27a; enterovirus; exosome secretion; nonstructural protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Sinev Iu V, Volotskov VI, Volkov SV. 1987. A catheter for laparoscopic drainage of the gallbladder. Khirurgiia (Mosk) 113–114. In Russian. - PubMed
-
- Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, Cameron CE, Ehrenfeld E, van Kuppeveld FJ, Altan-Bonnet N. 2010. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141:799–811. doi: 10.1016/j.cell.2010.03.050. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

