Characterization of a TatA/TatB binding site on the TatC component of the Escherichia coli twin arginine translocase
- PMID: 36790402
- PMCID: PMC10197872
- DOI: 10.1099/mic.0.001298
Characterization of a TatA/TatB binding site on the TatC component of the Escherichia coli twin arginine translocase
Abstract
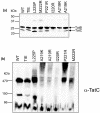
The twin arginine transport (Tat) pathway exports folded proteins across the cytoplasmic membranes of prokaryotes and the thylakoid membranes of chloroplasts. In Escherichia coli and other Gram-negative bacteria, the Tat machinery comprises TatA, TatB and TatC components. A Tat receptor complex, formed from all three proteins, binds Tat substrates, which triggers receptor organization and recruitment of further TatA molecules to form the active Tat translocon. The polytopic membrane protein TatC forms the core of the Tat receptor and harbours two binding sites for the sequence-related TatA and TatB proteins. A 'polar' cluster binding site, formed by TatC transmembrane helices (TMH) 5 and 6 is occupied by TatB in the resting receptor and exchanges for TatA during receptor activation. The second binding site, lying further along TMH6, is occupied by TatA in the resting state, but its functional relevance is unclear. Here we have probed the role of this second binding site through a programme of random and targeted mutagenesis. Characterization of three stably produced TatC variants, P221R, M222R and L225P, each of which is inactive for protein transport, demonstrated that the substitutions did not affect assembly of the Tat receptor. Moreover, the substitutions that we analysed did not abolish TatA or TatB binding to either binding site. Using targeted mutagenesis we introduced bulky substitutions into the TatA binding site. Molecular dynamics simulations and crosslinking analysis indicated that TatA binding at this site was substantially reduced by these amino acid changes, but TatC retained function. While it is not clear whether TatA binding at the TMH6 site is essential for Tat activity, the isolation of inactivating substitutions indicates that this region of the protein has a critical function.
Keywords: MD simulations; Tat pathway; TatC; mutagenesis; protein transport; twin arginine signal peptide.
Conflict of interest statement
The authors declare no conflict of interests
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- BB/S003339/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/S009213/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BB/R002517/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/P01948X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

