Worldwide Prevalence of Antibiotic-Associated Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis
- PMID: 36790777
- PMCID: PMC9932945
- DOI: 10.1001/jamadermatol.2022.6378
Worldwide Prevalence of Antibiotic-Associated Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis
Abstract
Importance: Antibiotics are an important risk for Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), which are the most severe types of drug hypersensitivity reaction with a mortality rate up to 50%. To our knowledge, no global systematic review has described antibiotic-associated SJS/TEN.
Objective: To evaluate the prevalence of antibiotics associated with SJS/TEN worldwide.
Data sources: The MEDLINE and Embase databases were searched for experimental and observational studies that described SJS/TEN risks since database inception to February 22, 2022.
Study selection: Included studies adequately described SJS/TEN origins and specified the antibiotics associated with SJS/TEN.
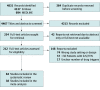
Data extraction and synthesis: Two reviewers (E.Y.L. and C.K.) independently selected the studies, extracted the data, and assessed the risk of bias. A meta-analysis using a random-effects model was performed in the studies that described patient-level associations. Subgroup analyses were performed to explore the heterogeneity. The risk of bias was assessed using the Joanna Briggs Institute checklist, and the certainty of evidence was rated using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach.
Main outcomes and measures: Prevalence of antibiotic-associated SJS/TEN was presented as pooled proportions with 95% CIs.
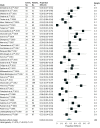
Results: Among the 64 studies included in the systematic review, there were 38 studies that described patient-level associations; the meta-analysis included these 38 studies with 2917 patients to determine the prevalence of single antibiotics associated with SJS/TEN. The pooled proportion of antibiotics associated with SJS/TEN was 28% (95% CI, 24%-33%), with moderate certainty of evidence. Among antibiotic-associated SJS/TEN, the sulfonamide class was associated with 32% (95% CI, 22%-44%) of cases, followed by penicillins (22%; 95% CI, 17%-28%), cephalosporins (11%; 95% CI, 6%-17%), fluoroquinolones (4%; 95% CI, 1%-7%), and macrolides (2%; 95% CI, 1%-5%). There was a statistically significant heterogeneity in the meta-analysis, which could be partially explained in the subgroup analysis by continents. The overall risk of bias was low using the Joanna Briggs Institute checklist for case series.
Conclusion and relevance: In this systematic review and meta-analysis of all case series, antibiotics were associated with more than one-quarter of SJS/TEN cases described worldwide, and sulfonamide antibiotics remained the most important association. These findings highlight the importance of antibiotic stewardship, clinician education and awareness, and weighing the risk-benefit assessment of antibiotic choice and duration.
Conflict of interest statement
Figures
References
-
- Mockenhaupt M, Dunant A, Paulmann M, et al. Drug causality in Stevens-Johnson syndrome/toxic epidermal necrolysis in Europe: analysis of 10 years RegiSCAR-Study. Pharmacoepidemiol Drug Saf. 2016;25(suppl 3):3.
-
- Shear N, Dodiuk-Gad R. Advances in Diagnosis and Management of Cutaneous Adverse Drug Reactions: Current and Future Trends. Adis; 2018.