Prevalence and Prognostic Significance of Bradyarrhythmias in Patients Screened for Atrial Fibrillation vs Usual Care: Post Hoc Analysis of the LOOP Randomized Clinical Trial
- PMID: 36790817
- PMCID: PMC9932940
- DOI: 10.1001/jamacardio.2022.5526
Prevalence and Prognostic Significance of Bradyarrhythmias in Patients Screened for Atrial Fibrillation vs Usual Care: Post Hoc Analysis of the LOOP Randomized Clinical Trial
Abstract
Importance: There is increasing interest in heart rhythm monitoring and technologies to detect subclinical atrial fibrillation (AF), which may lead to incidental diagnosis of bradyarrhythmias.
Objective: To assess bradyarrhythmia prevalence and prognostic significance in persons screened for AF using implantable loop recorder (ILR) compared with unscreened persons.
Design, setting, and participants: This was a post hoc analysis of the Implantable Loop Recorder Detection of Atrial Fibrillation to Prevent Stroke (LOOP) randomized clinical trial, which took place in 4 sites in Denmark. Participants were 70 years or older without known AF but diagnosed with at least 1 of the following: hypertension, diabetes, heart failure, or prior stroke. Participants were recruited by letter invitation between January 31, 2014, and May 17, 2016. The median (IQR) follow-up period was 65 (59-70) months. Analysis took place between February and June 2022.
Interventions: ILR screening for AF with treatment of any bradyarrhythmia left to the discretion of the treating physician (ILR group) vs usual care (control group).
Main outcomes and measures: Adjudicated bradyarrhythmia episodes, pacemaker implantation, syncope, and sudden cardiovascular death.
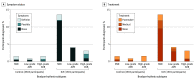
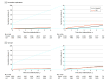
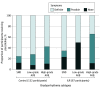
Results: A total of 6004 participants were randomized (mean [SD] age, 75 [4.1] years; 2837 [47.3%] female; 5444 [90.7%] with hypertension; 1224 [20.4%] with prior syncope), 4503 to control and 1501 to ILR. Bradyarrhythmia was diagnosed in 172 participants (3.8%) in the control group vs 312 participants (20.8%) in the ILR group (hazard ratio [HR], 6.21 [95% CI, 5.15-7.48]; P < .001), and these were asymptomatic in 41 participants (23.8%) vs 249 participants (79.8%), respectively. The most common bradyarrhythmia was sinus node dysfunction followed by high-grade atrioventricular block. Risk factors for bradyarrhythmia included higher age, male sex, and prior syncope. A pacemaker was implanted in 132 participants (2.9%) vs 67 (4.5%) (HR, 1.53 [95% CI, 1.14-2.06]; P < .001), syncope occurred in 120 (2.7%) vs 33 (2.2%) (HR, 0.83 [95% CI, 0.56-1.22]; P = .34), and sudden cardiovascular death occurred in 49 (1.1%) vs 18 (1.2%) (HR, 1.11 [95% CI, 0.64-1.90]; P = .71) in the control and ILR groups, respectively. Bradyarrhythmias were associated with subsequent syncope, cardiovascular death, and all-cause death, with no interaction between bradyarrhythmia and randomization group.
Conclusions and relevance: More than 1 in 5 persons older than 70 years with cardiovascular risk factors can be diagnosed with bradyarrhythmias when long-term continous monitoring for AF is applied. In this study, ILR screening led to a 6-fold increase in bradyarrhythmia diagnoses and a significant increase in pacemaker implantations compared with usual care but no change in the risk of syncope or sudden death.
Conflict of interest statement
Figures

Comment in
-
Incidental Detection of Bradycardia by Implantable Loop Recorders-Unintended Consequences.JAMA Cardiol. 2023 Apr 1;8(4):312-313. doi: 10.1001/jamacardio.2022.5541. JAMA Cardiol. 2023. PMID: 36790795 No abstract available.
References
-
- Lubitz SA, Faranesh AZ, Atlas SJ, et al. . Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the Fitbit heart study. Am Heart J. 2021;238:16-26. doi:10.1016/j.ahj.2021.04.003 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

