A Standardized Approach for Collection of Objective Data to Support Outcome Determination for Late-Phase Tuberculosis Clinical Trials
- PMID: 36790881
- PMCID: PMC10595436
- DOI: 10.1164/rccm.202206-1118OC
A Standardized Approach for Collection of Objective Data to Support Outcome Determination for Late-Phase Tuberculosis Clinical Trials
Abstract
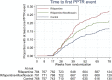
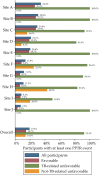
Rationale: We developed a standardized method, possible poor treatment response (PPTR), to help ascertain efficacy endpoints in Study S31/A5349 (NCT02410772), an open-label trial comparing two 4-month rifapentine-based regimens with a standard 6-month regimen for the treatment of pulmonary tuberculosis (TB). Objectives: We describe the use of the PPTR process and evaluate whether the goals of minimizing bias in efficacy endpoint assessment and attainment of relevant data to determine outcomes for all participants were achieved. Methods: A PPTR event was defined as the occurrence of one or more prespecified triggers. Each PPTR required initiation of a standardized evaluation process that included obtaining multiple sputum samples for microbiology. Measurements and Main Results: Among 2,343 participants with culture-confirmed drug-susceptible TB, 454 individuals (19.4%) had a total of 534 individual PPTR events, of which 76.6% were microbiological (positive smear or culture at or after 17 wk). At least one PPTR event was experienced by 92.4% (133 of 144) of participants with TB-related unfavorable outcome and between 13.8% and 14.7% of participants with favorable and not-assessable outcomes. A total of 75% of participants with TB-related unfavorable outcomes had microbiological confirmation of failure to achieve a disease-free cure. Conclusions: Standardized methodologies, such as our PPTR approach, could facilitate unbiased efficacy outcome determinations, improve discrimination between outcomes that are related and unrelated to regimen efficacy, and enhance the ability to conduct pooled analyses of contemporary trials.
Keywords: TB; endpoints ascertainment bias; multicenter randomized trial; noninferiority; outcomes.
Figures
Comment in
-
Ascertainment Bias in TB Treatment Trials: Can Systematic Assessment of Objective Endpoints Solve It?Am J Respir Crit Care Med. 2023 May 15;207(10):1269-1270. doi: 10.1164/rccm.202303-0430ED. Am J Respir Crit Care Med. 2023. PMID: 36952239 Free PMC article. No abstract available.
References
-
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER) Non-inferiority clinical trials to establish effectiveness: guidance for industry 2016. “https://www.fda.gov/media/78504/download” Non-Inferiority Clinical Trials to Establish Effectiveness Guidance for Industry (fda.gov)
-
- Phillips PPJ, Glidden DV. In: Principles and practice of clinical trials. Piantadosi S, Meinert CL, editors. Cham: Springer; 2021. Noninferiority trials.
-
- Dorman SE, Nahid P, Kurbatova EV, Goldberg SV, Bozeman L, Burman WJ, et al. AIDS Clinical Trials Group and the Tuberculosis Trials Consortium High-dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: study protocol for TBTC study 31/ACTG A5349 phase 3 clinical trial. Contemp Clin Trials . 2020;90:105938. - PMC - PubMed

