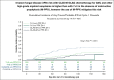
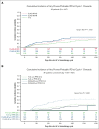
Conflict-of-interest disclosure: J.L. has served on advisory boards for Mayne Pharma, Merck Sharp & Dohme (MSD), Amgen, and Bristol Myers Squibb. M.A.S. has sat on advisory boards for Gilead, Pfizer, Merck, F2G, and Takeda, and received research funding from F2G, Gilead, and Merck. M.G. has served on advisory boards for Amgen, Pfizer, Servier, and Jazz Pharmaceuticals and has received trial and research support from Amgen and Servier outside of the submitted work. D.C.M.K. has served on advisory boards for Becton Dickinson Pty Ltd. and MSD and received financial support from MSD and F2G, all unrelated to the current work. S.A.P. receives research support from Global Life Technologies, Inc; participated in research trials with Chimerix Inc., Merck & Co, F2G, Cidara, and Symbio; and participated in a clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (U01-AI132004) in which vaccines were provided by Sanofi. M.B. served as a consultant for and received research funding from Gilead Sciences and Merck. A.B.H. served as a consultant for AbbVie and Agios and received research funding from Pfizer, Nohla Therapeutics, Jazz Pharmaceuticals, Imago Pharmaceuticals, Novartis, Bayer, Tolero Pharmaceuticals, Agios Pharmaceuticals, and Gilead. R.B.W. served as a consultant for AbbVie, Amgen, Amphivena, BerGenBio, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Immunogen, and Orum; received research funding from Celgene, Janssen, and Pfizer; and has ownership interests in Amphivena. J.A.H. has received a research grant from Deverra Therapeutics (formerly Nohla Therapeutics) for the support of the work; consulting fees from Karius Inc and Pfizer; and research support from Deverra Therapeutics, Karius, and Merck. The remaining authors declare no competing financial interests.