Cholesterol-rich domain formation mediated by ZO proteins is essential for tight junction formation
- PMID: 36791108
- PMCID: PMC9974431
- DOI: 10.1073/pnas.2217561120
Cholesterol-rich domain formation mediated by ZO proteins is essential for tight junction formation
Abstract
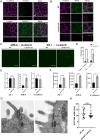
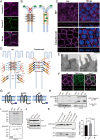
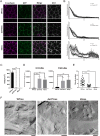
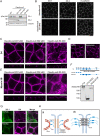
Tight junctions (TJs) are cell-adhesion structures responsible for the epithelial barrier. We reported that accumulation of cholesterol at the apical junctions is required for TJ formation [K. Shigetomi, Y. Ono, T. Inai, J. Ikenouchi, J. Cell Biol. 217, 2373-2381 (2018)]. However, it is unclear how cholesterol accumulates and informs TJ formation-and whether cholesterol enrichment precedes or follows the assembly of claudins in the first place. Here, we established an epithelial cell line (claudin-null cells) that lacks TJs by knocking out claudins. Despite the lack of TJs, cholesterol normally accumulated in the vicinity of the apical junctions. Assembly of claudins at TJs is thought to require binding to zonula occludens (ZO) proteins; however, a claudin mutant that cannot bind to ZO proteins still formed TJ strands. ZO proteins were however necessary for cholesterol accumulation at the apical junctions through their effect on the junctional actomyosin cytoskeleton. We propose that ZO proteins not only function as scaffolds for claudins but also promote TJ formation of cholesterol-rich membrane domains at apical junctions.
Keywords: cholesterol; claudin; epithelial cells; membrane domain; tight junction.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins.J Cell Biol. 1999 Dec 13;147(6):1351-63. doi: 10.1083/jcb.147.6.1351. J Cell Biol. 1999. PMID: 10601346 Free PMC article.
-
Role of claudin species-specific dynamics in reconstitution and remodeling of the zonula occludens.Mol Biol Cell. 2011 May;22(9):1495-504. doi: 10.1091/mbc.E10-12-1003. Epub 2011 Mar 3. Mol Biol Cell. 2011. PMID: 21372174 Free PMC article.
-
Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity.J Cell Biol. 2019 Oct 7;218(10):3372-3396. doi: 10.1083/jcb.201812157. Epub 2019 Aug 29. J Cell Biol. 2019. PMID: 31467165 Free PMC article.
-
Ruffles and spikes: Control of tight junction morphology and permeability by claudins.Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183339. doi: 10.1016/j.bbamem.2020.183339. Epub 2020 May 7. Biochim Biophys Acta Biomembr. 2020. PMID: 32389670 Free PMC article. Review.
-
The Claudins: From Tight Junctions to Biological Systems.Trends Biochem Sci. 2019 Feb;44(2):141-152. doi: 10.1016/j.tibs.2018.09.008. Epub 2018 Oct 25. Trends Biochem Sci. 2019. PMID: 30665499 Review.
Cited by
-
A short guide to the tight junction.J Cell Sci. 2024 May 1;137(9):jcs261776. doi: 10.1242/jcs.261776. Epub 2024 May 7. J Cell Sci. 2024. PMID: 38712627 Free PMC article. Review.
-
Storming the gate: New approaches for targeting the dynamic tight junction for improved drug delivery.Adv Drug Deliv Rev. 2023 Aug;199:114905. doi: 10.1016/j.addr.2023.114905. Epub 2023 Jun 3. Adv Drug Deliv Rev. 2023. PMID: 37271282 Free PMC article. Review.
-
Unraveling the link between neuropathy target esterase NTE/SWS, lysosomal storage diseases, inflammation, abnormal fatty acid metabolism, and leaky brain barrier.Elife. 2024 Apr 25;13:e98020. doi: 10.7554/eLife.98020. Elife. 2024. PMID: 38660940 Free PMC article.
-
Exploring the impact of lipid stress on sperm cytoskeleton: insights and prospects.Nat Rev Urol. 2025 May;22(5):294-312. doi: 10.1038/s41585-024-00952-1. Epub 2024 Nov 11. Nat Rev Urol. 2025. PMID: 39528754 Review.
-
Computational Models of Claudin Assembly in Tight Junctions and Strand Properties.Int J Mol Sci. 2024 Mar 16;25(6):3364. doi: 10.3390/ijms25063364. Int J Mol Sci. 2024. PMID: 38542338 Free PMC article. Review.
References
-
- Tsukita S., Furuse M., Itoh M., Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 (2001). - PubMed
-
- Umeda K., et al. , ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126, 741–754 (2006). - PubMed
-
- Beutel O., Maraspini R., Pombo-García K., Martin-Lemaitre C., Honigmann A., Phase separation of zonula occludens proteins drives formation of tight junctions. Cell 179, 923–936.e911 (2019). - PubMed
-
- Nusrat A., et al. , Tight junctions are membrane microdomains. J. Cell Sci. 113, 1771–1781 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- JP19H03227/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP21K19231/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP17J00211/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20K22632/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21444781/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources

