Data-driven longitudinal characterization of neonatal health and morbidity
- PMID: 36791208
- PMCID: PMC10197092
- DOI: 10.1126/scitranslmed.adc9854
Data-driven longitudinal characterization of neonatal health and morbidity
Abstract
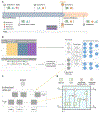
Although prematurity is the single largest cause of death in children under 5 years of age, the current definition of prematurity, based on gestational age, lacks the precision needed for guiding care decisions. Here, we propose a longitudinal risk assessment for adverse neonatal outcomes in newborns based on a deep learning model that uses electronic health records (EHRs) to predict a wide range of outcomes over a period starting shortly before conception and ending months after birth. By linking the EHRs of the Lucile Packard Children's Hospital and the Stanford Healthcare Adult Hospital, we developed a cohort of 22,104 mother-newborn dyads delivered between 2014 and 2018. Maternal and newborn EHRs were extracted and used to train a multi-input multitask deep learning model, featuring a long short-term memory neural network, to predict 24 different neonatal outcomes. An additional cohort of 10,250 mother-newborn dyads delivered at the same Stanford Hospitals from 2019 to September 2020 was used to validate the model. Areas under the receiver operating characteristic curve at delivery exceeded 0.9 for 10 of the 24 neonatal outcomes considered and were between 0.8 and 0.9 for 7 additional outcomes. Moreover, comprehensive association analysis identified multiple known associations between various maternal and neonatal features and specific neonatal outcomes. This study used linked EHRs from more than 30,000 mother-newborn dyads and would serve as a resource for the investigation and prediction of neonatal outcomes. An interactive website is available for independent investigators to leverage this unique dataset: https://maternal-child-health-associations.shinyapps.io/shiny_app/.
Conflict of interest statement
Figures


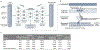


References
-
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP, Wyckoff M, Das A, Hale EC, Ball BB, Newman NS, Schibler K, Poindexter BB, Kennedy KA, Cotten CM, Watterberg KL, D’Angio CT, DeMauro SB, Truog WE, Devaskar U, Higgins RD, Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015). - PMC - PubMed
-
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, Watterberg KL, Saha S, Das A, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network, Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010). - PMC - PubMed
-
- Stevenson DK, Wong RJ, Hay WW Jr, Comments on the 20th anniversary of NeoReviews. Neoreviews 21, e643–e648 (2020). - PubMed

