Sex-dimorphic hindbrain lactate regulation of ventromedial hypothalamic nucleus glucoregulatory neuron 5'-AMP-activated protein kinase activity and transmitter marker protein expression
- PMID: 36791640
- PMCID: PMC10175150
- DOI: 10.1016/j.npep.2023.102324
Sex-dimorphic hindbrain lactate regulation of ventromedial hypothalamic nucleus glucoregulatory neuron 5'-AMP-activated protein kinase activity and transmitter marker protein expression
Abstract
Background: The oxidizable glycolytic end-product L-lactate is a gauge of nerve cell metabolic fuel stability that metabolic-sensory hindbrain A2 noradrenergic neurons impart to the brain glucose-regulatory network. Current research investigated the premise that hindbrain lactate deficiency exerts sex-specific control of energy sensor and transmitter marker protein responses to hypoglycemia in ventromedial hypothalamic nucleus (VMN) glucose-regulatory nitrergic and γ-aminobutyric acid (GABA) neurons.
Methods: Nitric oxide synthase (nNOS)- or glutamate decarboxylase65/67 (GAD)-immunoreactive neurons were laser-catapult-microdissected from male and female rat VMN after subcutaneous insulin injection and caudal fourth ventricular L-lactate or vehicle infusion for Western blot protein analysis.
Results: Hindbrain lactate repletion reversed hypoglycemia-associated augmentation (males) or inhibition (females) of nitrergic neuron nNOS expression, and prevented up-regulation of phosphorylated AMPK 5'-AMP-activated protein kinase (pAMPK) expression in those neurons. Hypoglycemic suppression of GABAergic neuron GAD protein was averted by exogenous lactate over the rostro-caudal length of the male VMN and in the middle region of the female VMN. Lactate normalized GABA neuron pAMPK profiles in hypoglycemic male (caudal VMN) and female (all VMN segments) rats. Hypoglycemic patterns of norepinephrine (NE) signaling were lactate-dependent throughout the male VMN, but confined to the rostral and middle female VMN.
Conclusions: Results document, in each sex, regional VMN glucose-regulatory transmitter responses to hypoglycemia that are controlled by hindbrain lactate status. Hindbrain metabolic-sensory regulation of hypoglycemia-correlated nitric oxide or GABA release may entail AMPK-dependent mechanisms in specific VMN rostro-caudal segments in each sex. Additional effort is required to examine the role of hindbrain lactoprivic-sensitive VMN neurotransmitters in lactate-mediated attenuation of hypoglycemic hyperglucagonemia and hypercorticosteronemia in male and female rats.
Keywords: AMPK; Corticosterone; L-lactate; Nitric oxide; Norepinephrine; Sex differences.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflicts of interests to declare.
Figures


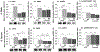

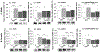



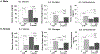
Similar articles
-
Impact of caudal hindbrain glycogen metabolism on A2 noradrenergic neuron AMPK activation and ventromedial hypothalamic nucleus norepinephrine activity and glucoregulatory neurotransmitter marker protein expression.Neuropeptides. 2020 Aug;82:102055. doi: 10.1016/j.npep.2020.102055. Epub 2020 May 16. Neuropeptides. 2020. PMID: 32451071 Free PMC article.
-
Sex-Dimorphic Octadecaneuropeptide (ODN) Regulation of Ventromedial Hypothalamic Nucleus Glucoregulatory Neuron Function and Counterregulatory Hormone Secretion.ASN Neuro. 2023 Jan-Dec;15:17590914231167230. doi: 10.1177/17590914231167230. ASN Neuro. 2023. PMID: 37194319 Free PMC article.
-
G protein-coupled lactate receptor GPR81 control of ventrolateral ventromedial hypothalamic nucleus glucoregulatory neurotransmitter and 5'-AMP-activated protein kinase expression.Am J Physiol Regul Integr Comp Physiol. 2023 Jan 1;324(1):R20-R34. doi: 10.1152/ajpregu.00100.2022. Epub 2022 Nov 21. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36409024 Free PMC article.
-
Norepinephrine Regulation of Ventromedial Hypothalamic Nucleus Metabolic-Sensory Neuron 5'-AMP-Activated Protein Kinase Activity: Impact of Estradiol.Int J Mol Sci. 2020 Mar 16;21(6):2013. doi: 10.3390/ijms21062013. Int J Mol Sci. 2020. PMID: 32188013 Free PMC article. Review.
-
Norepinephrine Regulation of Ventromedial Hypothalamic Nucleus Astrocyte Glycogen Metabolism.Int J Mol Sci. 2021 Jan 13;22(2):759. doi: 10.3390/ijms22020759. Int J Mol Sci. 2021. PMID: 33451134 Free PMC article. Review.
Cited by
-
Sex-dependent endozepinergic regulation of ventromedial hypothalamic nucleus glucose counter-regulatory neuron aromatase protein expression in the adult rat.J Chem Neuroanat. 2023 Oct;132:102323. doi: 10.1016/j.jchemneu.2023.102323. Epub 2023 Aug 3. J Chem Neuroanat. 2023. PMID: 37543285 Free PMC article.
-
Aging Effects on Absolute and Relative Estrogen Receptor Variant Gene Expression Levels in Male Versus Female Rat Ventromedial Hypothalamic Nucleus Growth Hormone-Releasing Hormone Neurons.J Integr Neurosci. 2025 Jun 23;24(6):38142. doi: 10.31083/JIN38142. J Integr Neurosci. 2025. PMID: 40613376 Free PMC article.
References
-
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem 1997; 272: 30096–30102. doi: 10.1074/jbc.272.48.30096. - DOI - PubMed
-
- Patil GD, Briski KP. Lactate is a critical ‘sensed’ variable in caudal hindbrain monitoring of CNS metabolic stasis. Amer. J. Physiol. Regul. Integr. Comp. Physiol 2005; 289: R1777–86. - PubMed
-
- Vavaiya KV, Briski KP. Effects of caudal hindbrain lactate infusion on insulin-induced hypoglycemia and neuronal substrate transporter glucokinase and sulfonylurea receptor-1 gene expression in the ovariectomized female rat dorsal vagal complex: Impact of estradiol. J. Neurosci Res 2008; 86(3): 694–701. doi: 10.1002/jnr.21530. - DOI - PubMed
-
- Vavaiya KV, Briski KP. Caudal hindbrain lactate infusion alters glucokinase, SUR1, and neuronal substrate fuel transporter gene expression in the dorsal vagal complex, lateral hypothalamic area, and ventromedial nucleus hypothalamus of hypoglycemic male rats. Brain Res 2007; 1176: 62–70. doi: 10.1016/j.brainres.2007.08.010. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

