Efficacy of Steroid-Impregnated Spacers After Endoscopic Sinus Surgery in Chronic Rhinosinusitis: A Systematic Review and Meta-Analysis
- PMID: 36791807
- PMCID: PMC10208850
- DOI: 10.21053/ceo.2022.01718
Efficacy of Steroid-Impregnated Spacers After Endoscopic Sinus Surgery in Chronic Rhinosinusitis: A Systematic Review and Meta-Analysis
Abstract
Objectives: The aim of this study was to compare the effect of steroid-impregnated spacers to that of conventional management after endoscopic sinus surgery (ESS) in patients with chronic rhinosinusitis (CRS).
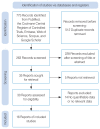
Methods: Six databases were searched from inception until November 2022. Sixteen studies were found that compared the improvement of chronic sinusitis-related symptoms and postoperative outcomes between a steroid-impregnated spacer group and a control group (non-steroid-impregnated spacers). The Cochrane risk of bias tool (for randomized controlled studies) and the Newcastle-Ottawa Scale (for non-randomized controlled studies) were used to assess the quality of the works included.
Results: Regarding the endoscopic findings, the degree of mucosal edema, ethmoid inflammation, crust formation at 2-3 months postoperatively, nasal discharge, polyposis, and scarring/synechia were significantly lower in the steroid-impregnated spacer group. The steroid-impregnated spacer group also showed significantly lower Lund-Kennedy scores and perioperative sinus endoscopy scores than the control group at 2-3 weeks postoperatively. Furthermore, the steroid-impregnated spacer group had lower rates of adhesions, middle turbinate lateralization, polypoid changes, the need for oral steroid use, the need for postoperative therapeutic interventions, and lysis of adhesions than controls. However, no significant between-group differences were found in short-term (2-3 weeks postoperatively) endoscopic findings regarding nasal discharge, postoperative crusting, polyposis, or scarring/synechia.
Conclusion: Steroid-impregnated nasal packing reduced the rates of postoperative intervention and recurrent polyposis and inflammation in CRS patients undergoing ESS.
Keywords: Nose; Operative Surgical Procedures; Sinusitis; Stents; Steroids.
Conflict of interest statement
Sung Won Kim and Do Hyun Kim are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Figures


References
-
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020 Feb;58(Suppl 29):1–464. - PubMed
-
- Orlandi RR, Kingdom TT, Smith TL, Bleier B, DeConde A, Luong AU, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021 Mar;11(3):213–739. - PubMed
-
- Svider PF, Sekhsaria V, Cohen DS, Eloy JA, Setzen M, Folbe AJ. Geographic and temporal trends in frontal sinus surgery. Int Forum Allergy Rhinol. 2015 Jan;5(1):46–54. - PubMed

