Interstitial cells of Cajal in gastrointestinal inflammatory diseases
- PMID: 36792171
- PMCID: PMC9926098
- DOI: 10.1540/jsmr.59.1
Interstitial cells of Cajal in gastrointestinal inflammatory diseases
Abstract
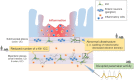
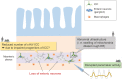
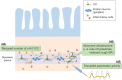
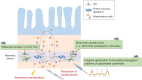
The gastrointestinal (GI) tract is a vital organ that digests food, absorbs nutrients, and excretes waste. Normal GI motility is the basis for these functions. The interstitial cells of Cajal (ICC) in the GI muscularis layer promote GI motility together with the enteric nervous system and smooth muscle cells. Since GI motility results from complex coordination of these heterogeneous cells, failure of any one of them can lead to GI dysmotility. Knowledge about ICC in physiological conditions has accumulated in recent decades, while the pathophysiology of ICC in GI inflammatory diseases, such as inflammatory bowel disease, is not well understood. In this review, we summarize the previous studies about the pathophysiological changes of ICC in inflammatory diseases and discuss the inflammatory mediators that induce ICC dysfunction.
Keywords: gastrointestinal motility; inflammation; interstitial cells of Cajal.
Figures
References
-
- Cajal SR y Histologie du système nerveux de l’homme & des vertébrés: Cervelet, cerveau moyen, rétine, couche optique, corps strié, écorce cérébrale générale & régionale, grand sympathique. Vol 2. A. Maloine; 1911.
-
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, et al. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 1991; 10(8): 2111–8. doi: 10.1002/j.1460-2075.1991.tb07744.x - DOI - PMC - PubMed
-
- Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995; 280(1): 97–111. - PubMed

