Imaging Voltage Globally and in Isofrequency Lamina in Slices of Mouse Ventral Cochlear Nucleus
- PMID: 36792362
- PMCID: PMC9997695
- DOI: 10.1523/ENEURO.0465-22.2023
Imaging Voltage Globally and in Isofrequency Lamina in Slices of Mouse Ventral Cochlear Nucleus
Abstract
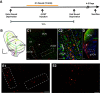
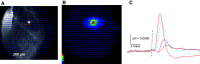
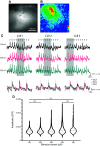
The cochlear nuclei (CNs) receive sensory information from the ear and perform fundamental computations before relaying this information to higher processing centers. These computations are performed by distinct types of neurons interconnected in circuits dedicated to the specialized roles of the auditory system. In the present study, we explored the use of voltage imaging to investigate CN circuitry. We tested two approaches based on fundamentally different voltage sensing technologies. Using a voltage-sensitive dye we recorded glutamate receptor-independent signals arising predominantly from axons. The mean conduction velocity of these fibers of 0.27 m/s was rapid but in range with other unmyelinated axons. We then used a genetically-encoded hybrid voltage sensor (hVOS) to image voltage from a specific population of neurons. Probe expression was controlled using Cre recombinase linked to c-fos activation. This activity-induced gene enabled targeting of neurons that are activated when a mouse hears a pure 15-kHz tone. In CN slices from these animals auditory nerve fiber stimulation elicited a glutamate receptor-dependent depolarization in hVOS probe-labeled neurons. These cells resided within a band corresponding to an isofrequency lamina, and responded with a high degree of synchrony. In contrast to the axonal origin of voltage-sensitive dye signals, hVOS signals represent predominantly postsynaptic responses. The introduction of voltage imaging to the CN creates the opportunity to investigate auditory processing circuitry in populations of neurons targeted on the basis of their genetic identity and their roles in sensory processing.
Keywords: auditory nerve; c-fos; cochlear nuclei; genetically-encoded voltage sensors; tonotopic organization; voltage imaging.
Copyright © 2023 Ma et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Imaging Voltage in Genetically Defined Neuronal Subpopulations with a Cre Recombinase-Targeted Hybrid Voltage Sensor.J Neurosci. 2017 Sep 20;37(38):9305-9319. doi: 10.1523/JNEUROSCI.1363-17.2017. Epub 2017 Aug 23. J Neurosci. 2017. PMID: 28842412 Free PMC article.
-
Nitric Oxide-Mediated Plasticity of Interconnections Between T-Stellate cells of the Ventral Cochlear Nucleus Generate Positive Feedback and Constitute a Central Gain Control in the Auditory System.J Neurosci. 2019 Jul 31;39(31):6095-6107. doi: 10.1523/JNEUROSCI.0177-19.2019. Epub 2019 Jun 3. J Neurosci. 2019. PMID: 31160538 Free PMC article.
-
Functional Development of the Auditory Brainstem Nuclei During Embryogenesis of the Mouse Revealed by Optical Recording With a Voltage-Sensitive Dye.Eur J Neurosci. 2025 Apr;61(7):e70106. doi: 10.1111/ejn.70106. Eur J Neurosci. 2025. PMID: 40219726
-
The role of intrinsic neuronal properties in the encoding of auditory information in the cochlear nuclei.Curr Opin Neurobiol. 1991 Aug;1(2):221-8. doi: 10.1016/0959-4388(91)90082-i. Curr Opin Neurobiol. 1991. PMID: 1821185 Review.
-
The Cochlear Spiral Ganglion Neurons: The Auditory Portion of the VIII Nerve.Anat Rec (Hoboken). 2019 Mar;302(3):463-471. doi: 10.1002/ar.23815. Epub 2018 May 4. Anat Rec (Hoboken). 2019. PMID: 29659185 Review.
Cited by
-
High calcium concentrations reduce cellular excitability of mouse MNTB neurons.Brain Res. 2023 Dec 1;1820:148568. doi: 10.1016/j.brainres.2023.148568. Epub 2023 Sep 7. Brain Res. 2023. PMID: 37689332 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
