Association of Higher Ocrelizumab Exposure With Reduced Disability Progression in Multiple Sclerosis
- PMID: 36792367
- PMCID: PMC9931184
- DOI: 10.1212/NXI.0000000000200094
Association of Higher Ocrelizumab Exposure With Reduced Disability Progression in Multiple Sclerosis
Erratum in
-
Association of Higher Ocrelizumab Exposure With Reduced Disability Progression in Multiple Sclerosis.Neurol Neuroimmunol Neuroinflamm. 2023 Mar 27;10(3):e200120. doi: 10.1212/NXI.0000000000200120. Print 2023 May. Neurol Neuroimmunol Neuroinflamm. 2023. PMID: 36973077 Free PMC article. No abstract available.
Abstract
Background and objectives: Ocrelizumab improved clinical and MRI measures of disease activity and progression in three phase 3 multiple sclerosis (MS) studies. Post hoc analyses demonstrated a correlation between the ocrelizumab serum concentration and the degree of blood B-cell depletion, and body weight was identified as the most influential covariate on ocrelizumab pharmacokinetics. The magnitude of ocrelizumab treatment benefit on disability progression was greater in lighter vs heavier patients. These observations suggest that higher ocrelizumab serum levels provide more complete B-cell depletion and a greater delay in disability progression. The current post hoc analyses assessed population exposure-efficacy/safety relationships of ocrelizumab in patients with relapsing and primary progressive MS.
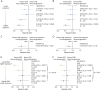
Methods: Patients in OPERA I/II and ORATORIO were grouped in exposure quartiles based on their observed individual serum ocrelizumab level over the treatment period. Exposure-response relationships were analyzed for clinical efficacy (24-week confirmed disability progression (CDP), annualized relapse rate [ARR], and MRI outcomes) and adverse events.
Results: Ocrelizumab reduced new MRI lesion counts to nearly undetectable levels in patients with relapsing or primary progressive MS across all exposure subgroups, and reduced ARR in patients with relapsing MS to very low levels (0.13-0.18). A consistent trend of higher ocrelizumab exposure leading to lower rates of CDP was seen (0%-25% [lowest] to 75%-100% [highest] quartile hazard ratios and 95% confidence intervals; relapsing MS: 0.70 [0.41-1.19], 0.85 [0.52-1.39], 0.47 [0.25-0.87], and 0.34 [0.17-0.70] vs interferon β-1a; primary progressive MS: 0.88 [0.59-1.30], 0.86 [0.60-1.25], 0.77 [0.52-1.14], and 0.55 [0.36-0.83] vs placebo). Infusion-related reactions, serious adverse events, and serious infections were similar across exposure subgroups.
Discussion: The almost complete reduction of ARR and MRI activity already evident in the lowest quartile, and across all ocrelizumab-exposure groups, suggests a ceiling effect. A consistent trend of higher ocrelizumab exposure leading to greater reduction in risk of CDP was observed, particularly in the relapsing MS trials, and was not associated with a higher rate of adverse events. Higher ocrelizumab exposure may provide improved control of disability progression by reducing disease activity below that detectable by ARR and MRI, and/or by attenuating other B-cell-related pathologies responsible for tissue damage.
Classification of evidence: This analysis provides Class III evidence that higher ocrelizumab serum levels are related to greater reduction in risk of disability progression in patients with multiple sclerosis. The study is rated Class III because of the initial treatment randomization disclosure that occurred after inclusion in the open-label extension.
Trial registration information: ClinicalTrials.gov Identifier: NCT01247324 (OPERA I), NCT01412333 (OPERA II), and NCT01194570 (ORATORIO).
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
-
- Wolinsky JS, Arnold DL, Brochet B, et al. . Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19(12):998-1009. doi: 10.1016/S1474-4422(20)30342-2. - DOI - PubMed
