Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3-C3aR signalling
- PMID: 36792546
- PMCID: PMC9931688
- DOI: 10.1002/jev2.12306
Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3-C3aR signalling
Abstract
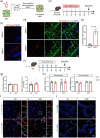
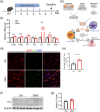
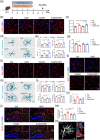
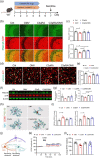
The gut microbiota represents a diverse and dynamic population of microorganisms that can influence the health of the host. Increasing evidence supports the role of the gut microbiota as a key player in the pathogenesis of neurodegenerative diseases, including Alzheimer's disease (AD). Unfortunately, the mechanisms behind the interplay between gut pathogens and AD are still elusive. It is known that bacteria-derived outer membrane vesicles (OMVs) act as natural carriers of virulence factors that are central players in the pathogenesis of the bacteria. Helicobacter pylori (H. pylori) is a common gastric pathogen and H. pylori infection has been associated with an increased risk to develop AD. Here, we are the first to shed light on the role of OMVs derived from H. pylori on the brain in healthy conditions and on disease pathology in the case of AD. Our results reveal that H. pylori OMVs can cross the biological barriers, eventually reaching the brain. Once in the brain, these OMVs are taken up by astrocytes, which induce activation of glial cells and neuronal dysfunction, ultimately leading to exacerbated amyloid-β pathology and cognitive decline. Mechanistically, we identified a critical role for the complement component 3 (C3)-C3a receptor (C3aR) signalling in mediating the interaction between astrocytes, microglia and neurons upon the presence of gut H. pylori OMVs. Taken together, our study reveals that H. pylori has a detrimental effect on brain functionality and accelerates AD development via OMVs and C3-C3aR signalling.
Keywords: Helicobacter pylori; Alzheimer's disease; C3; bacterial extracellular vesicles (bEVs); complement; gut-brain axis; outer membrane vesicles (OMVs).
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Albaret, G. , Sifré, E. , Floch, P. , Laye, S. , Aubert, A. , Dubus, P. , Azzi‐Martin, L. , Giese, A. , Salles, N. , Mégraud, F. , Varon, C. , Lehours, P. , & Roubaud‐Baudron, C. (2020). Alzheimer's disease and Helicobacter pylori infection: Inflammation from stomach to brain? Journal of Alzheimers Disease, 73, 801–809. - PubMed
-
- Bartels, T. , De Schepper, S. , & Hong, S. (2020). Microglia modulate neurodegeneration in Alzheimer's and Parkinson's diseases. Science, 370, 66–69. - PubMed
-
- Berstad, A. E. , Høgåsen, K. , Bukholm, G. , Moran, A. P. , & Brandtzaeg, P. (2001). Complement activation directly induced by Helicobacter pylori . Gastroenterology, 120, 1108–1116. - PubMed
-
- Bielaszewska, M. , Rüter, C. , Bauwens, A. , Greune, L. , Jarosch, K.‐A. , Steil, D. , Zhang, W. , He, X. , Lloubes, R. , Fruth, A. , Kim, K. S. , Schmidt, M. A. , Dobrindt, U. , Mellmann, A. , & Karch, H. (2017). Host cell interactions of outer membrane vesicle‐associated virulence factors of enterohemorrhagic Escherichia coli O157: Intracellular delivery, trafficking and mechanisms of cell injury. PLoS Pathogens, 13, e1006159. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

