DNALI1 deficiency causes male infertility with severe asthenozoospermia in humans and mice by disrupting the assembly of the flagellar inner dynein arms and fibrous sheath
- PMID: 36792588
- PMCID: PMC9932082
- DOI: 10.1038/s41419-023-05653-y
DNALI1 deficiency causes male infertility with severe asthenozoospermia in humans and mice by disrupting the assembly of the flagellar inner dynein arms and fibrous sheath
Abstract
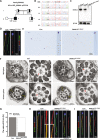
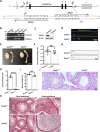
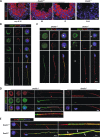
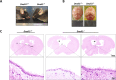
The axonemal dynein arms (outer (ODA) and inner dynein arms (IDAs)) are multiprotein structures organized by light, intermediate, light intermediate (LIC), and heavy chain proteins. They hydrolyze ATP to promote ciliary and flagellar movement. Till now, a variety of dynein protein deficiencies have been linked with asthenospermia (ASZ), highlighting the significance of these structures in human sperm motility. Herein, we detected bi-allelic DNALI1 mutations [c.663_666del (p.Glu221fs)], in an ASZ patient, which resulted in the complete loss of the DNALI1 in the patient's sperm. We identified loss of sperm DNAH1 and DNAH7 rather than DNAH10 in both DNALI1663_666del patient and Dnali1-/- mice, demonstrating that mammalian DNALI1 is a LIC protein of a partial IDA subspecies. More importantly, we revealed that DNALI1 loss contributed to asymmetries in the most fibrous sheath (FS) of the sperm flagellum in both species. Immunoprecipitation revealed that DNALI1 might interact with the cytoplasmic dynein complex proteins in the testes. Furthermore, DNALI1 loss severely disrupted the transport and assembly of the FS proteins, especially AKAP3 and AKAP4, during flagellogenesis. Hence, DNALI1 may possess a non-classical molecular function, whereby it regulates the cytoplasmic dynein complex that assembles the flagella. We conclude that a DNALI deficiency-induced IDAs injury and an asymmetric FS-driven tail rigid structure alteration may simultaneously cause flagellum immotility. Finally, intracytoplasmic sperm injection (ICSI) can effectively resolve patient infertility. Collectively, we demonstrate that DNALI1 is a newly causative gene for AZS in both humans and mice, which possesses multiple crucial roles in modulating flagellar assembly and motility.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
DNAH3 deficiency causes flagellar inner dynein arm loss and male infertility in humans and mice.Elife. 2024 Nov 6;13:RP96755. doi: 10.7554/eLife.96755. Elife. 2024. PMID: 39503742 Free PMC article.
-
Deficiency in DNAH12 causes male infertility by impairing DNAH1 and DNALI1 recruitment in humans and mice.Elife. 2025 Mar 27;13:RP100350. doi: 10.7554/eLife.100350. Elife. 2025. PMID: 40146200 Free PMC article.
-
Homozygous mutation in DNALI1 leads to asthenoteratozoospermia by affecting the inner dynein arms.Front Endocrinol (Lausanne). 2023 Jan 16;13:1058651. doi: 10.3389/fendo.2022.1058651. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36726469 Free PMC article.
-
Genetic underpinnings of asthenozoospermia.Best Pract Res Clin Endocrinol Metab. 2020 Dec;34(6):101472. doi: 10.1016/j.beem.2020.101472. Epub 2020 Nov 6. Best Pract Res Clin Endocrinol Metab. 2020. PMID: 33191078 Review.
-
Novel DNAH1 Mutation Loci Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Literature Review.World J Mens Health. 2022 Oct;40(4):551-560. doi: 10.5534/wjmh.210119. Epub 2022 Jan 25. World J Mens Health. 2022. PMID: 35118838 Free PMC article. Review.
Cited by
-
Unveiling the therapeutic potential: KBU2046 halts triple-negative breast cancer cell migration by constricting TGF-β1 activation in vitro.Oncol Res. 2024 Oct 16;32(11):1791-1802. doi: 10.32604/or.2024.049348. eCollection 2024. Oncol Res. 2024. PMID: 39449805 Free PMC article.
-
Genetic Causes of Qualitative Sperm Defects: A Narrative Review of Clinical Evidence.Genes (Basel). 2024 May 8;15(5):600. doi: 10.3390/genes15050600. Genes (Basel). 2024. PMID: 38790229 Free PMC article. Review.
-
Adenylate kinase phosphate energy shuttle underlies energetic communication in flagellar axonemes.Sci China Life Sci. 2024 Aug;67(8):1697-1714. doi: 10.1007/s11427-023-2539-1. Epub 2024 May 16. Sci China Life Sci. 2024. PMID: 38761355
-
Function of manchette and intra-manchette transport in spermatogenesis and male fertility.Cell Commun Signal. 2025 May 29;23(1):250. doi: 10.1186/s12964-025-02213-z. Cell Commun Signal. 2025. PMID: 40442757 Free PMC article. Review.
-
AXDND1 is required to balance spermatogonial commitment and for sperm tail formation in mice and humans.Cell Death Dis. 2024 Jul 12;15(7):499. doi: 10.1038/s41419-024-06874-5. Cell Death Dis. 2024. PMID: 38997255 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

