Anti-DEspR antibody treatment improves survival and reduces neurologic deficits in a hypertensive, spontaneous intracerebral hemorrhage (hsICH) rat model
- PMID: 36792616
- PMCID: PMC9932093
- DOI: 10.1038/s41598-023-28149-3
Anti-DEspR antibody treatment improves survival and reduces neurologic deficits in a hypertensive, spontaneous intracerebral hemorrhage (hsICH) rat model
Abstract
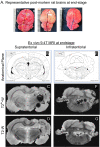
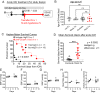
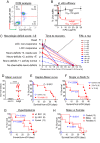
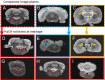
Progressive secondary brain injury-induced by dysregulated neuroinflammation in spontaneous intracerebral hemorrhage (sICH)-underlies high sICH-mortality and remains without FDA-approved pharmacotherapy. Clinical insight that hematoma-directed interventions do not improve mortality prioritizes resolving acute secondary brain injury in sICH. As neutrophils are implicated in sICH secondary brain injury, we tested whether inhibition of a rogue neutrophil-subset expressing the dual endothelin-1/signal peptide receptor (DEspR) and associated with secondary tissue injury, DEspR+ CD11b+ immunotype, will attenuate mortality in a hypertensive-sICH (hsICH) rat model. We confirmed sICH-related deaths in hsICH-rats by T2*-weighted 9.4 T MRI and DEspR+ neutrophils in hsICH-rat brain perihematomal areas by immunostaining. At acute sICH, anti-DEspR muIgG1-antibody, mu10a3, treatment increased median survival in hsICH rats vs controls (p < 0.0001). In pre-stroke sICH, weekly 10a3-treatment did not predispose to infection and delayed sICH-onset vs controls (p < 0.0001). As potential sICH-therapeutic, we tested humanized anti-DEspR IgG4S228P-mAb, hu6g8. In vitro, hu6g8 reversed delayed-apoptosis in DEspR+ CD11b+ neutrophils. In vivo, hu6g8 increased median survival and reduced neurologic symptoms in male/female hsICH-rats vs controls (p < 0.0001). Altogether, preclinical efficacy of inhibition of DEspR+ CD11b+ neutrophils in acute sICH-without infection complications, supports the potential of anti-DEspR therapy in sICH. Data provide basis for clinical study of DEspR+ CD11b+ neutrophil-subset in sICH patients.
© 2023. The Author(s).
Conflict of interest statement
VLMH and NRO: co-inventors on BU patents on DEspR and DEspR-inhibition for stroke therapy. All other authors have no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

