Developmental mechanisms underlying the evolution of human cortical circuits
- PMID: 36792753
- PMCID: PMC10064077
- DOI: 10.1038/s41583-023-00675-z
Developmental mechanisms underlying the evolution of human cortical circuits
Abstract
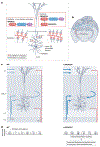
The brain of modern humans has evolved remarkable computational abilities that enable higher cognitive functions. These capacities are tightly linked to an increase in the size and connectivity of the cerebral cortex, which is thought to have resulted from evolutionary changes in the mechanisms of cortical development. Convergent progress in evolutionary genomics, developmental biology and neuroscience has recently enabled the identification of genomic changes that act as human-specific modifiers of cortical development. These modifiers influence most aspects of corticogenesis, from the timing and complexity of cortical neurogenesis to synaptogenesis and the assembly of cortical circuits. Mutations of human-specific genetic modifiers of corticogenesis have started to be linked to neurodevelopmental disorders, providing evidence for their physiological relevance and suggesting potential relationships between the evolution of the human brain and its sensitivity to specific diseases.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


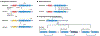
References
-
- Lichtman JW & Denk W The big and the small: challenges of imaging the brain’s circuits.Science 334, 618–623 (2011). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

