Generating human blastoids modeling blastocyst-stage embryos and implantation
- PMID: 36792779
- PMCID: PMC7617227
- DOI: 10.1038/s41596-023-00802-1
Generating human blastoids modeling blastocyst-stage embryos and implantation
Abstract
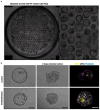
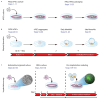
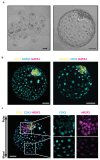
Human early development sets the stage for embryonic and adult life but remains difficult to investigate. A solution came from the ability of stem cells to organize into structures resembling preimplantation embryos-blastocysts-that we termed blastoids. This embryo model is available in unlimited numbers and could thus support scientific and medical advances. However, its predictive power depends on how faithfully it recapitulates the blastocyst. Here, we describe how we formed human blastoids that (1) efficiently achieve the morphology of the blastocyst and (2) form lineages according to the pace and sequence of blastocyst development, (3) ultimately forming cells that transcriptionally reflect the blastocyst (preimplantation stage). We employ three different commercially available 96- and 24-well microwell plates with results similar to our custom-made ones, and show that blastoids form in clinical in vitro fertilization medium and can be cryopreserved for shipping. Finally, we explain how blastoids replicate the directional process of implantation into endometrial organoids, specifically when these are hormonally stimulated. It takes 4 d for human blastoids to form and 10 d to prepare the endometrial implantation assay, and we have cultured blastoids up to 6 d (time-equivalent of day 13). On the basis of our experience, we anticipate that a person with ~1 year of human pluripotent stem cell culture experience and of organoid culture should be able to perform the protocol. Altogether, blastoids offer an opportunity to establish scientific and biomedical discovery programs for early pregnancy, and an ethical alternative to the use of embryos.
© 2023. Springer Nature Limited.
Conflict of interest statement
The Institute for Molecular Biotechnology, Austrian Academy of Sciences has filed patent application EP21151455.9 describing the protocols for human blastoid formation and for the blastoid–endometrium interaction assay. H.K., A.J., H.H.K. and N.R. are the inventors on this patent. All other authors declare no competing interests.
Figures







References
-
- Stamatiadis P, et al. TEAD4 regulates trophectoderm differentiation upstream of CDX2 in a GATA3-independent manner in the human preimplantation embryo. Human Reproduction. 2022 - PubMed
-
- Ma H, et al. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science. 2019;366 - PubMed
-
- O’Leary T, et al. Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat Biotechnol. 2012;30:278–282. - PubMed
-
- Sunde A, et al. Time to take human embryo culture seriously. Hum Reprod. 2016;31:2174–2182. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

