Reactive Oxygen Species and Ferroptosis at the Nexus of Inflammation and Colon Cancer
- PMID: 36792928
- PMCID: PMC10517337
- DOI: 10.1089/ars.2023.0246
Reactive Oxygen Species and Ferroptosis at the Nexus of Inflammation and Colon Cancer
Abstract
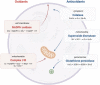
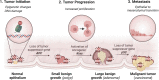
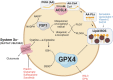
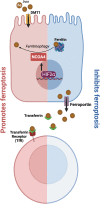
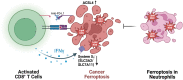
Significance: Reactive oxygen species (ROS) are essential in maintaining normal intestinal physiology. Inflammatory bowel disease (IBD) is a relapsing chronic inflammatory disease of the intestine that is a major risk factor for colorectal cancer (CRC). Excess ROS are widely implicated in intestinal inflammation and cancer. Recent Advances: Clinical data have shown that targeting ROS broadly does not yield improved outcomes in IBD and CRC. However, selectively limiting oxidative damage may improve the efficacy of ROS targeting. An accumulation of lipid ROS induces a novel oxidative cell death pathway known as ferroptosis. A growing body of evidence suggests that ferroptosis is relevant to both IBD and CRC. Critical Issues: We propose that inhibition of ferroptosis will improve disease severity in IBD, whereas activating ferroptosis will limit CRC progression. Data from preclinical models suggest that methods of modulating ferroptosis have been successful in attenuating IBD and CRC. Future Directions: The etiology of IBD and progression of IBD to CRC are still unclear. Further understanding of ferroptosis in intestinal diseases will provide novel therapies. Ferroptosis is highly linked to inflammation, cell metabolism, and is cell-type dependent. Further research in assessing the inflammatory and tumor microenvironment in the intestine may provide novel vulnerabilities that can be targeted. Antioxid. Redox Signal. 39, 551-568.
Keywords: CRC; IBD; colorectal cancer; ferroptosis; inflammatory bowel disease.
Conflict of interest statement
No competing financial interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

