Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment
- PMID: 36793048
- PMCID: PMC9930367
- DOI: 10.1186/s12943-023-01722-0
Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment
Abstract
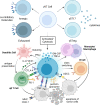
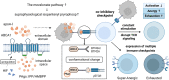
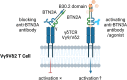
As a nontraditional T-cell subgroup, γδT cells have gained popularity in the field of immunotherapy in recent years. They have extraordinary antitumor potential and prospects for clinical application. Immune checkpoint inhibitors (ICIs), which are efficacious in tumor patients, have become pioneer drugs in the field of tumor immunotherapy since they were incorporated into clinical practice. In addition, γδT cells that have infiltrated into tumor tissues are found to be in a state of exhaustion or anergy, and there is upregulation of many immune checkpoints (ICs) on their surface, suggesting that γδT cells have a similar ability to respond to ICIs as traditional effector T cells. Studies have shown that targeting ICs can reverse the dysfunctional state of γδT cells in the tumor microenvironment (TME) and exert antitumor effects by improving γδT-cell proliferation and activation and enhancing cytotoxicity. Clarification of the functional state of γδT cells in the TME and the mechanisms underlying their interaction with ICs will solidify ICIs combined with γδT cells as a good treatment option.
Keywords: Antitumor immunotherapy; Checkpoint inhibitor (CPI); Immune checkpoint blockade (ICB); Immune checkpoint inhibitors (ICIs); Immune checkpoint molecules; Immune checkpoint therapy (ICT); Tumor microenvironment (TME); γδT cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Immune checkpoints in the tumor microenvironment.Semin Cancer Biol. 2020 Oct;65:1-12. doi: 10.1016/j.semcancer.2019.06.021. Epub 2019 Jun 29. Semin Cancer Biol. 2020. PMID: 31265893 Review.
-
Target tumor microenvironment by innate T cells.Front Immunol. 2022 Oct 6;13:999549. doi: 10.3389/fimmu.2022.999549. eCollection 2022. Front Immunol. 2022. PMID: 36275727 Free PMC article. Review.
-
Tumor-infiltrating gamma delta T-cells reveal exhausted subsets with remarkable heterogeneity in colorectal cancer.Int J Cancer. 2023 Nov 1;153(9):1684-1697. doi: 10.1002/ijc.34669. Epub 2023 Aug 2. Int J Cancer. 2023. PMID: 37531161
-
The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies.Front Immunol. 2020 May 7;11:784. doi: 10.3389/fimmu.2020.00784. eCollection 2020. Front Immunol. 2020. PMID: 32457745 Free PMC article. Review.
-
Resistance to immune checkpoint inhibitors and the tumor microenvironment.Exp Dermatol. 2023 Mar;32(3):240-249. doi: 10.1111/exd.14716. Epub 2022 Dec 8. Exp Dermatol. 2023. PMID: 36437644 Review.
Cited by
-
Developing a machine learning-based prognosis and immunotherapeutic response signature in colorectal cancer: insights from ferroptosis, fatty acid dynamics, and the tumor microenvironment.Front Immunol. 2024 Jul 15;15:1416443. doi: 10.3389/fimmu.2024.1416443. eCollection 2024. Front Immunol. 2024. PMID: 39076986 Free PMC article.
-
Non-Classical HLA Class 1b and Hepatocellular Carcinoma.Biomedicines. 2023 Jun 9;11(6):1672. doi: 10.3390/biomedicines11061672. Biomedicines. 2023. PMID: 37371767 Free PMC article. Review.
-
The Bidirectional Interplay between T Cell-Based Immunotherapies and the Tumor Microenvironment.Cancer Immunol Res. 2025 Apr 2;13(4):463-475. doi: 10.1158/2326-6066.CIR-24-0857. Cancer Immunol Res. 2025. PMID: 39786986 Free PMC article. Review.
-
A Pan-Cancer Study of Tumour-Associated Efferocytosis Core Genes and Preliminary Exploration of TIMD4 in Renal Cell Carcinoma.J Cell Mol Med. 2025 Jun;29(12):e70671. doi: 10.1111/jcmm.70671. J Cell Mol Med. 2025. PMID: 40576208 Free PMC article.
-
Effective γδ T-cell clinical therapies: current limitations and future perspectives for cancer immunotherapy.Clin Transl Immunology. 2024 Feb 19;13(2):e1492. doi: 10.1002/cti2.1492. eCollection 2024. Clin Transl Immunology. 2024. PMID: 38375329 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

